Indicate whether each statement is true or false. (a) ΔS is a state function. (b) If a system undergoes a reversible change, the entropy of the universe increases. (c) If a system undergoes a reversible process, the change in entropy of the system is exactly matched by an equal and opposite change in the entropy of the surroundings. (d) If a system undergoes a reversible process, the entropy change of the system must be zero.

The normal boiling point of Br2(𝑙) is 58.8 °C, and its molar enthalpy of vaporization is Δ𝐻vap=29.6kJ/mol. (b) Calculate the value of Δ𝑆 when 1.00 mol of Br2(𝑙) is vaporized at 58.8 °C.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Enthalpy of Vaporization
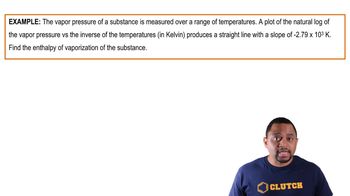
Entropy (ΔS)

Temperature Conversion
The normal boiling point of Br2(l) is 58.8 °C, and its molar enthalpy of vaporization is ΔHvap = 29.6 kJ/mol. (a) When Br2(l) boils at its normal boiling point, does its entropy increase or decrease?
The element gallium (Ga) freezes at 29.8 °C, and its molar enthalpy of fusion is ΔHfus = 5.59 kJ/mol. (a) When molten gallium solidifies to Ga(s) at its normal melting point, is ΔS positive or negative?
The element gallium (Ga) freezes at 29.8 °C, and its molar enthalpy of fusion is ΔHfus = 5.59 kJ/mol. (b) Calculate the value of ΔS when 60.0 g of Ga(l) solidifies at 29.8 °C.
Indicate whether each statement is true or false. (c) In a certain spontaneous process the system undergoes an entropy change of 4.2 J/K; therefore, the entropy change of the surroundings must be -4.2 J/K.
