Predict the sign of ΔSsys for each of the following processes: (d) Calcium phosphate precipitates upon mixing Ca(NO3)2(aq) and (NH4)3PO4(aq).
Ch.19 - Chemical Thermodynamics

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 19, Problem 48
Predict which member of each of the following pairs has the greater standard entropy at 25°C: (a) C6H6(l) or C6H6(g). Use Appendix C to find the standard entropy of each substance. (b) CO(g) or CO2(g). Use Appendix C to find the standard entropy of each substance. (c) 1 mol N2O4(g) or 2 mol NO2(g). Use Appendix C to find the standard entropy of each substance. (d) HCl(g) or HCl(aq). Use Appendix C to find the standard entropy of each substance.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of standard entropy. Standard entropy (S°) is a measure of the amount of disorder or randomness in a system at a standard state, typically 1 atm pressure and 25°C. Gases generally have higher entropy than liquids or solids due to greater molecular motion and disorder.
Step 2: For part (a), compare C6H6(l) and C6H6(g). Recognize that the gaseous state (C6H6(g)) will have a higher entropy than the liquid state (C6H6(l)) because gas molecules are more disordered and have more freedom of movement.
Step 3: For part (b), compare CO(g) and CO2(g). Consider the molecular complexity and number of atoms. CO2(g) has more atoms and a more complex structure than CO(g), which generally leads to higher entropy.
Step 4: For part (c), compare 1 mol N2O4(g) and 2 mol NO2(g). Consider the number of moles and molecular complexity. 2 mol NO2(g) will have higher entropy than 1 mol N2O4(g) because there are more moles of gas, leading to greater disorder.
Step 5: For part (d), compare HCl(g) and HCl(aq). Recognize that gases typically have higher entropy than aqueous solutions because gas molecules are more disordered than molecules in solution.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Standard Entropy
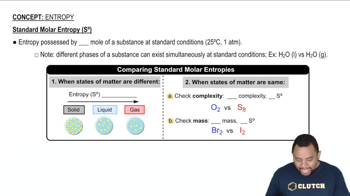
Standard entropy is a measure of the disorder or randomness of a system at a specified temperature, typically 25°C. It is denoted as S° and is expressed in units of J/(mol·K). Higher entropy values indicate greater disorder, which often correlates with the number of microstates available to a substance. Understanding standard entropy is crucial for predicting the spontaneity of reactions and the behavior of substances in different states.
Recommended video:
Guided course
Standard Molar Entropy
Phase Changes and Entropy
The phase of a substance significantly affects its entropy. Gases generally have higher entropy than liquids and solids due to their greater freedom of movement and higher number of accessible microstates. For example, comparing liquid and gaseous forms of the same substance, the gas will typically exhibit a higher standard entropy. This concept is essential for predicting which member of a pair will have greater entropy based on their physical states.
Recommended video:
Guided course

Entropy in Phase Changes
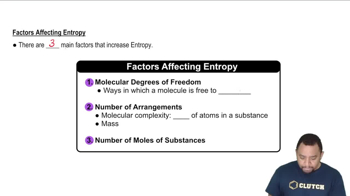
Molecular Complexity and Entropy
The complexity of a molecule, including its size and the number of atoms, influences its entropy. Larger molecules with more atoms can have more vibrational, rotational, and translational modes, leading to higher entropy values. When comparing different gases or molecular species, understanding how molecular structure affects entropy is vital for making accurate predictions about their standard entropy values.
Recommended video:
Guided course
Factors Affecting Entropy
Related Practice
Textbook Question
Textbook Question
Cyclopropane and propylene are isomers that both have the formula C3H6. Based on the molecular structures shown, which of these isomers would you expect to have the higher standard molar entropy at 25 °C?
Textbook Question
Using S° values from Appendix C, calculate ΔS° values for the following reactions. In each case, account for the sign of ΔS°.
(a) C2H4(g) + H2(g) → C2H6(g)
(b) N2O4(g) → 2 NO2(g)
(c) Be(OH)2(s) → BeO(s) + H2O(g)
(d) 2 CH3OH(g) + 3 O2(g) ⟶ 2 CO2(g) + 4 H2O(g)
Textbook Question
(a) For a process that occurs at constant temperature, does the change in Gibbs free energy depend on changes in the enthalpy and entropy of the system?
1
views
