Textbook Question
(a) In a chemical reaction, two gases combine to form a solid. What do you expect for the sign of ΔS?

 Verified step by step guidance
Verified step by step guidance
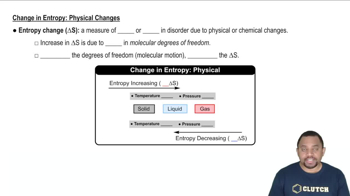

(a) In a chemical reaction, two gases combine to form a solid. What do you expect for the sign of ΔS?
(b) How does the entropy of the system change in the processes described in Exercise 19.12?
Indicate whether each statement is true or false. (a) Unlike enthalpy, where we can only ever know changes in H, we can know absolute values of S. (b) If you heat a gas such as CO2, you will increase its degrees of translational, rotational and vibrational motions. (c) CO2(g) and Ar(g) have nearly the same molar mass. At a given temperature, they will have the same number of microstates.