(d) Does the amount of work that a system can do on its surroundings depend on the path of the process?
Ch.19 - Chemical Thermodynamics

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 19, Problem 15c
Consider the vaporization of liquid water to steam at a pressure of 1 atm. (c) In what temperature range is it a nonspontaneous process?
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Identify the process: Vaporization of liquid water to steam at 1 atm pressure.
insert step 2> Recall that a process is nonspontaneous when the change in Gibbs free energy (ΔG) is positive.
insert step 3> Use the Gibbs free energy equation: ΔG = ΔH - TΔS, where ΔH is the enthalpy change, T is the temperature in Kelvin, and ΔS is the entropy change.
insert step 4> Recognize that at the boiling point of water (100°C or 373 K at 1 atm), ΔG = 0, meaning the process is at equilibrium.
insert step 5> Conclude that the process is nonspontaneous at temperatures below 100°C, where ΔG > 0, because the vaporization requires energy input to overcome intermolecular forces.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Gibbs Free Energy
Gibbs Free Energy (G) is a thermodynamic potential that helps predict the spontaneity of a process at constant temperature and pressure. A process is spontaneous if the change in Gibbs Free Energy (ΔG) is negative. For the vaporization of water, understanding how temperature affects ΔG is crucial in determining the conditions under which the process becomes nonspontaneous.
Recommended video:
Guided course
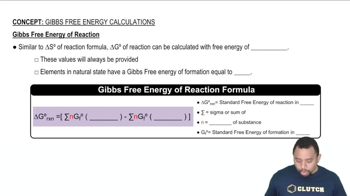
Gibbs Free Energy of Reactions
Phase Equilibrium
Phase equilibrium refers to the state where the rates of vaporization and condensation of a substance are equal, resulting in no net change in the amount of liquid and vapor. At 1 atm, water vaporizes at 100°C. Below this temperature, the equilibrium shifts towards the liquid phase, indicating that vaporization is nonspontaneous as the system favors condensation.
Recommended video:
Guided course
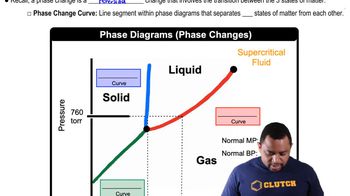
Phase Changes in Diagrams
Clausius-Clapeyron Equation
The Clausius-Clapeyron equation describes the relationship between the pressure and temperature of a substance during phase changes. It provides insight into how the vapor pressure of water changes with temperature, which is essential for understanding the conditions under which vaporization occurs. This equation helps identify the temperature range where vaporization is nonspontaneous by relating it to the pressure of the system.
Recommended video:
Guided course
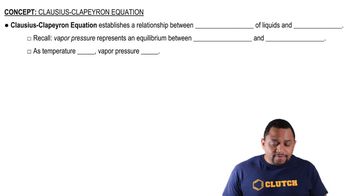
Clausius-Clapeyron Equation
Related Practice
Textbook Question
2
views
Textbook Question
Consider the vaporization of liquid water to steam at a pressure of 1 atm. (a) Is this process endothermic or exothermic?
1
views
Textbook Question
Consider the vaporization of liquid water to steam at a pressure of 1 atm. (b) In what temperature range is it a spontaneous process?
Textbook Question
Consider the vaporization of liquid water to steam at a pressure of 1 atm. (d) At what temperature are the two phases in equilibrium?
Textbook Question
The normal freezing point of n-octane (C8H18) is -57 °C. (b) In what temperature range is the freezing of n-octane a spontaneous process?
Textbook Question
The normal freezing point of n-octane (C8H18) is -57 °C. (d) Is there any temperature at which liquid n-octane and solid n-octane are in equilibrium? Explain.
