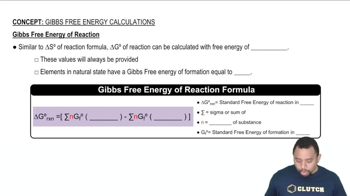
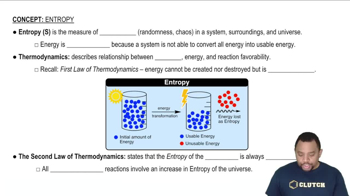
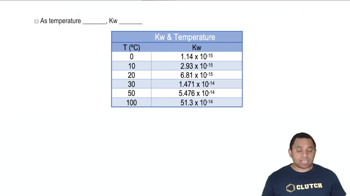
Using data from Appendix C, calculate the change in Gibbs free energy for each of the following reactions. In each case, indicate whether the reaction is spontaneous at 298 K under standard conditions.
(a) 2 Ag(s) + Cl2(g) → 2 AgCl(s)
(b) P4O10(s) + 16 H2(g) → 4 PH3(g) + 10 H2O(g)
(c) CH4(g) + 4 F2(g) → CF4(g) + 4 HF(g)
(d) 2 H2O2(l) → 2 H2O(l) + O2(g)