Textbook Question
For the isothermal expansion of a gas into a vacuum, ΔE = 0, q = 0, and w = 0. (b) Explain why no work is done by the system during this process.

 Verified step by step guidance
Verified step by step guidance

For the isothermal expansion of a gas into a vacuum, ΔE = 0, q = 0, and w = 0. (b) Explain why no work is done by the system during this process.
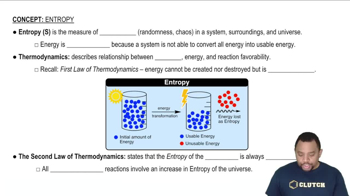
(a) What is the difference between a state and a microstate of a system?
(b) As a system goes from state A to state B, its entropy decreases. What can you say about the number of microstates corresponding to each state?
Would each of the following changes increase, decrease, or have no effect on the number of microstates available to a system: (b) decrease in volume
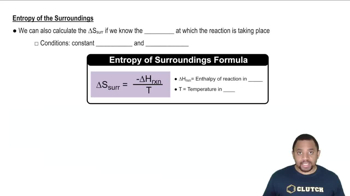
(a) In a chemical reaction, two gases combine to form a solid. What do you expect for the sign of ΔS?