For a certain chemical reaction, ΔH° = -35.4 kJ and ΔS° = -85.5 J/K. (b) Does the reaction lead to an increase or decrease in the randomness or disorder of the system?

A certain reaction has ΔH° = +23.7 kJ and ΔS° = +52.4 J>K. (c) Calculate ΔG° for the reaction at 298 K. (d) Is the reaction spontaneous at 298 K under standard conditions?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Gibbs Free Energy (ΔG)
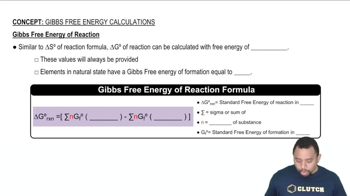
Enthalpy (ΔH)

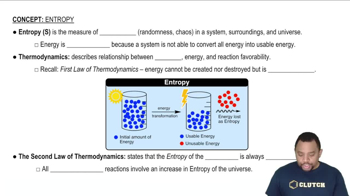
Entropy (ΔS)
For a certain chemical reaction, ΔH° = -35.4 kJ and ΔS° = -85.5 J/K. (c) Calculate ΔG° for the reaction at 298 K. (d) Is the reaction spontaneous at 298 K under standard conditions?
Use data in Appendix C to calculate ΔH°, ΔS°, and ΔG° at 25 °C for each of the following reactions.
a. 4 Cr(s) + 3 O2(g) → 2 Cr2O3(s)
b. BaCO3(s) → BaO(s) + CO2(g)
c. 2 P(s) + 10 HF(g) → 2 PF5(g) + 5 H2(g)
d. K(s) + O2(g) → KO2(s)
Using data from Appendix C, calculate ΔG° for the following reactions. Indicate whether each reaction is spontaneous at 298 K under standard conditions.
(a) 2 SO2(g) + O2(g) → 2 SO3(g)
(b) NO2(g) + N2O(g) → 3 NO(g)
(c) 6 Cl2(g) + 2 Fe2O3(s) → 4 FeCl3(s) + 3 O2(g)
(d) SO2(g) + 2 H2(g) → S(s) + 2 H2O(g)
Using data from Appendix C, calculate the change in Gibbs free energy for each of the following reactions. In each case, indicate whether the reaction is spontaneous at 298 K under standard conditions.
(a) 2 Ag(s) + Cl2(g) → 2 AgCl(s)
(b) P4O10(s) + 16 H2(g) → 4 PH3(g) + 10 H2O(g)
(c) CH4(g) + 4 F2(g) → CF4(g) + 4 HF(g)
(d) 2 H2O2(l) → 2 H2O(l) + O2(g)
