A solution containing several metal ions is treated with dilute HCl; no precipitate forms. The pH is adjusted to about 1, and H2S is bubbled through. Again, no precipitate forms. The pH of the solution is then adjusted to about 8. Again, H2S is bubbled through. This time a precipitate forms. The filtrate from this solution is treated with (NH4)2HPO4. No precipitate forms. Which of these metal cations are either possibly present or definitely absent: Al3+, Na+, Ag+, Mg2+?
Ch.17 - Additional Aspects of Aqueous Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 17, Problem 79a,b
In the course of various qualitative analysis procedures, the following mixtures are encountered: (a) Zn2+ and Cd2+. (b) Cr(OH)3 and Fe(OH)3 Suggest how each mixture might be separated.
 Verified step by step guidance
Verified step by step guidance1
Identify the chemical properties of Zn^{2+} and Cd^{2+} ions, such as their solubility in different reagents.
Consider using a reagent that forms a precipitate with one ion but not the other. For example, sulfide ions (S^{2-}) can be used to precipitate CdS, which is insoluble, while ZnS is more soluble in acidic conditions.
Add a dilute acid to the mixture to ensure that ZnS remains dissolved while CdS precipitates out.
Filter the mixture to separate the solid CdS from the solution containing Zn^{2+}.
Wash and dry the precipitate to obtain pure CdS, and the remaining solution will contain Zn^{2+} ions.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility and Precipitation Reactions
Solubility refers to the ability of a substance to dissolve in a solvent, while precipitation reactions occur when two soluble salts react to form an insoluble compound. Understanding these concepts is crucial for separating ions in a mixture, as selective precipitation can be used to isolate specific ions based on their differing solubilities.
Recommended video:
Guided course

Selective Precipitation
Complex Ion Formation
Complex ion formation involves the interaction of metal ions with ligands to form a stable complex. This concept is essential for separating metal ions like Zn<sup>2+</sup> and Cd<sup>2+</sup>, as specific ligands can selectively bind to one metal ion over the other, allowing for their separation through techniques such as extraction or chromatography.
Recommended video:
Guided course
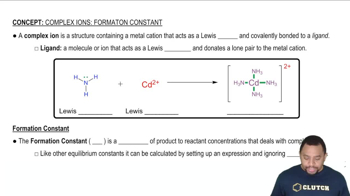
Complex Ions and Formation Constant
Ion Selectivity and Chromatography
Ion selectivity refers to the ability to preferentially separate ions based on their chemical properties. Chromatography is a technique that exploits these differences, allowing for the separation of Zn<sup>2+</sup> and Cd<sup>2+</sup> by passing the mixture through a medium that interacts differently with each ion, thus achieving separation based on their affinities.
Recommended video:
Guided course
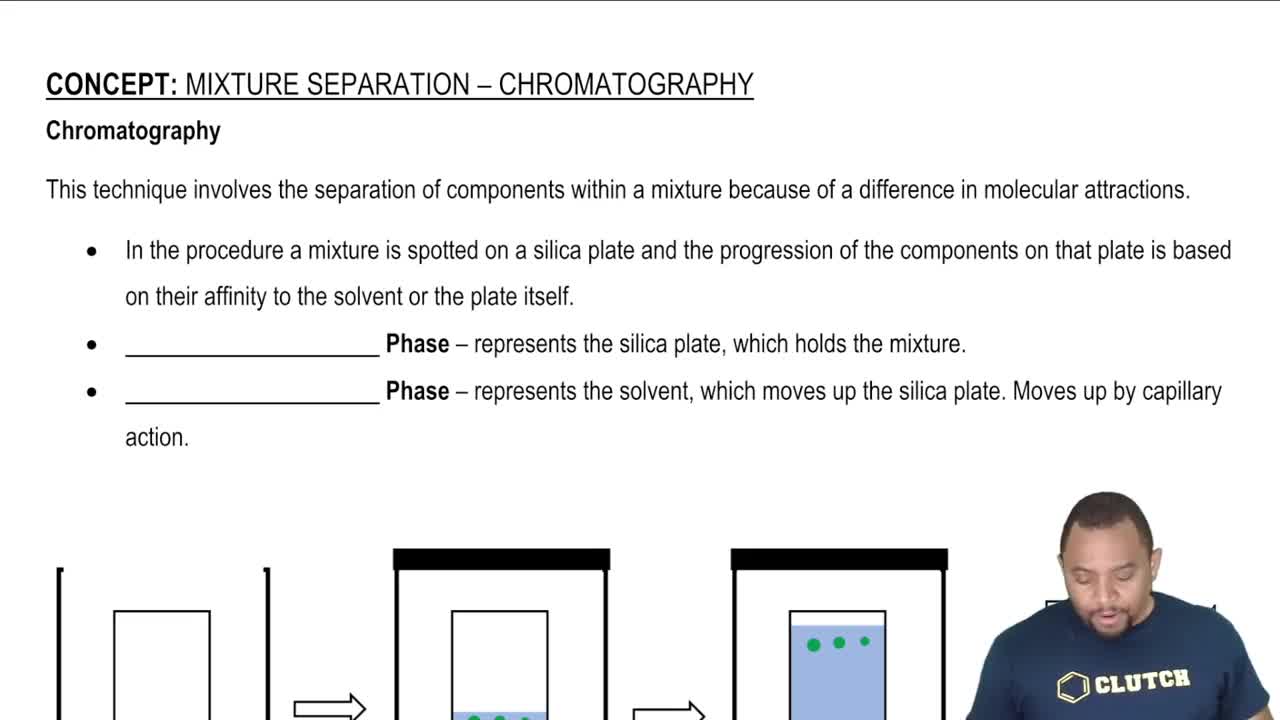
Chromatography
Related Practice
Textbook Question
1
views
Textbook Question
In the course of various qualitative analysis procedures, the following mixtures are encountered: (c) Mg2+ and K+ (d) Ag+ and Mn2+. Suggest how each mixture might be separated.
1
views
Textbook Question
Suggest how the cations in each of the following solution mixtures can be separated: (c) Pb2 + and Al3 +.
1
views
Textbook Question
(b) What is the most significant difference between the sulfides precipitated in group 2 and those precipitated in group 3?
