(b) What is the most significant difference between the sulfides precipitated in group 2 and those precipitated in group 3?

Furoic acid (HC5H3O3) has a Ka value of 6.76 × 10^-4 at 25 _x001F_C. Calculate the pH at 25 _x001F_C of (a) a solution formed by adding 25.0 g of furoic acid and 30.0 g of sodium furoate (NaC5H3O3) to enough water to form 0.250 L of solution; (b) a solution formed by mixing 30.0 mL of 0.250 M HC5H3O3 and 20.0 mL of 0.22 M NaC5H3O3 and diluting the total volume to 125 mL; (c) a solution prepared by adding 50.0 mL of 1.65 M NaOH solution to 0.500 L of 0.0850 M HC5H3O3.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
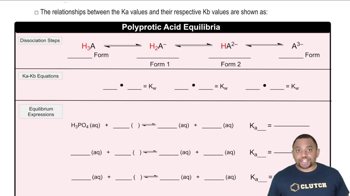
Acid-Base Equilibrium
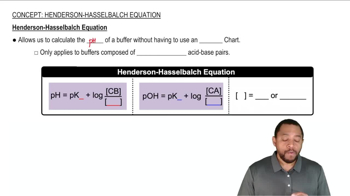
Henderson-Hasselbalch Equation
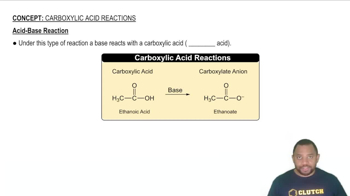
Stoichiometry in Acid-Base Reactions
Which of these equations relates the pOH of a buffer to the p𝐾𝑏 of its weak base, analogous to the Henderson–Hasselbalch equation for weak acids?
a. p𝐾𝑏=pOH+p𝐾𝑏=pOH+log[acid]/[base]
b. p𝐾𝑏=pOH−log[acid]/[base]
c. p𝐾𝑏=pOH−log[base]/[acid]
d. p𝐾𝑏=pOH+log[base]/[[acid]
Rainwater is acidic because CO21g2 dissolves in the water, creating carbonic acid, H2CO3. If the rainwater is too acidic, it will react with limestone and seashells (which are principally made of calcium carbonate, CaCO3). Calculate the concentrations of carbonic acid, bicarbonate ion 1HCO3-2 and carbonate ion 1CO32 - 2 that are in a raindrop that has a pH of 5.60, assuming that the sum of all three species in the raindrop is 1.0 * 10-5 M.
The acid–base indicator bromcresol green is a weak acid. The yellow acid and blue base forms of the indicator are present in equal concentrations in a solution when the pH is 4.68. What is the pKa for bromcresol green?
Two buffers are prepared by adding an equal number of moles of formic acid (HCOOH) and sodium formate (HCOONa) to enough water to make 1.00 L of solution. Buffer A is prepared using 1.00 mol each of formic acid and sodium formate. Buffer B is prepared by using 0.010 mol of each. (b) Which buffer will have the greater buffer capacity?
