A 20.0-mL sample of 0.200 M HBr solution is titrated with 0.200 M NaOH solution. Calculate the pH of the solution after the following volumes of base have been added:
(a) 15.0 mL
(b) 19.9 mL.

 Verified step by step guidance
Verified step by step guidance
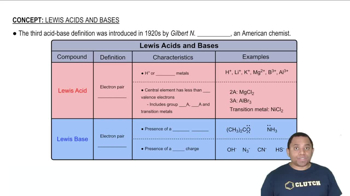
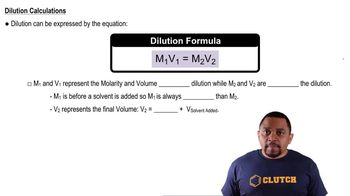

A 20.0-mL sample of 0.200 M HBr solution is titrated with 0.200 M NaOH solution. Calculate the pH of the solution after the following volumes of base have been added:
(a) 15.0 mL
(b) 19.9 mL.
A 20.0-mL sample of 0.200 M HBr solution is titrated with 0.200 M NaOH solution. Calculate the pH of the solution after the following volumes of base have been added:
(c) 20.0 mL.
A 20.0-mL sample of 0.200 M HBr solution is titrated with 0.200 M NaOH solution. Calculate the pH of the solution after the following volumes of base have been added: (e) 35.0 mL.
A 20.0-mL sample of 0.150 M KOH is titrated with 0.125 M HClO4 solution. Calculate the pH after the following volumes of acid have been added: (c) 24.0 mL.
A 20.0-mL sample of 0.150 M KOH is titrated with 0.125 M HClO4 solution. Calculate the pH after the following volumes of acid have been added: (d) 25.0 mL. (e) 30.0 mL.
A 35.0-mL sample of 0.150 M acetic acid 1CH3COOH2 is titrated with 0.150 M NaOH solution. Calculate the pH after the following volumes of base have been added: (b) 17.5 mL.