Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (d) Cl-

Which of the following is the stronger Brønsted–Lowry acid, HClO3 or HClO2?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Brønsted–Lowry Acid-Base Theory

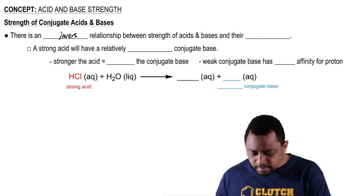
Acid Strength and Conjugate Bases
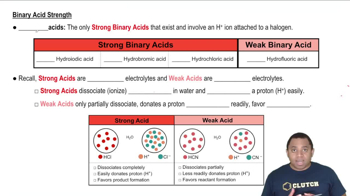
Oxidation State and Acid Strength
Label each of the following as being a strong base, a weak base, or a species with negligible basicity. In each case write the formula of its conjugate acid, and indicate whether the conjugate acid is a strong acid, a weak acid, or a species with negligible acidity: (e) NH3.
Which of the following is the stronger Brønsted–Lowry acid, HBrO or HBr?
Predict the products of the following acid–base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow:
(a) O2-(aq) + H2O(l) ⇌
(b) CH3COOH(aq) + HS-(aq) ⇌
(c) NO2-(aq) + H2O(l) ⇌
Predict the products of the following acid–base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow:
(a) NH4+(aq) + OH-(aq) ⇌
(b) CH3COO-(aq) + H3O+(aq) ⇌
(c) HCO3-(aq) + F-(aq) ⇌
