Citric acid, which is present in citrus fruits, is a triprotic acid (Table 16.3). (a) Calculate the pH of a 0.040 M solution of citric acid. (b) Did you have to make any approximations or assumptions in completing your calculations? (c) Is the concentration of citrate ion 1C6H5O7 3-2 equal to, less than, or greater than the H+ ion concentration?
Ch.16 - Acid-Base Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 16, Problem 72c
The hypochlorite ion, ClO-, acts as a weak base. (b) When ClO- acts as a base, which atom, Cl or O, acts as the proton acceptor? (c) Can you use formal charges to rationalize your answer to part (b)?
 Verified step by step guidance
Verified step by step guidance1
insert step 1> Identify the role of the hypochlorite ion (ClO-) as a base, which means it will accept a proton (H+).
insert step 2> Consider the structure of the hypochlorite ion: Cl is bonded to O, and O has a lone pair of electrons.
insert step 3> Determine which atom, Cl or O, is more likely to accept a proton. Oxygen is more electronegative than chlorine and has lone pairs, making it a better candidate for proton acceptance.
insert step 4> Use formal charges to rationalize: In ClO-, the formal charge on O is typically -1, while Cl is 0. The negative charge on O indicates a higher electron density, making it more likely to attract and accept a proton.
insert step 5> Conclude that the oxygen atom in ClO- acts as the proton acceptor due to its higher electronegativity and negative formal charge.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Base Theory
Acid-base theory explains the behavior of substances in terms of proton transfer. According to the Brønsted-Lowry definition, an acid is a proton donor, while a base is a proton acceptor. In the case of the hypochlorite ion (ClO-), understanding its role as a weak base is crucial for determining which atom accepts the proton during a reaction.
Recommended video:
Guided course
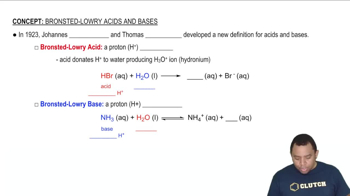
Bronsted-Lowry Acid-Base Theory
Proton Acceptor
In the context of acid-base reactions, the proton acceptor is the species that gains a hydrogen ion (H+). For the hypochlorite ion, the oxygen atom is typically the site of protonation due to its higher electronegativity compared to chlorine. This property allows oxygen to stabilize the positive charge that results from accepting a proton, making it the more likely proton acceptor.
Recommended video:
Guided course
Neutron-to-Proton Plot
Formal Charge
Formal charge is a theoretical concept used to determine the distribution of electrons in a molecule. It is calculated by comparing the number of valence electrons in an atom to the number of electrons it owns in a Lewis structure. In the case of ClO-, analyzing formal charges can help rationalize which atom (Cl or O) is more stable after protonation, thus supporting the identification of the proton acceptor.
Recommended video:
Guided course
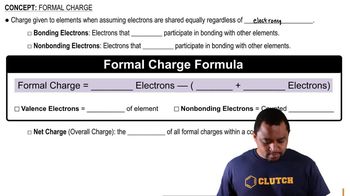
Formal Charge
Related Practice
Textbook Question
Textbook Question
Consider the base hydroxylamine, NH2OH. (a) What is the conjugate acid of hydroxylamine?
Textbook Question
The hypochlorite ion, ClO-, acts as a weak base. (a) Is ClO- a stronger or weaker base than hydroxylamine?
Textbook Question
Write the chemical equation and the Kb expression for the reaction of each of the following bases with water: (a) propylamine, C3H7NH2
Textbook Question
Write the chemical equation and the Kb expression for the reaction of each of the following bases with water: (c) benzoate ion, C6H5CO2-
