Textbook Question
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (b) NaBr

 Verified step by step guidance
Verified step by step guidance

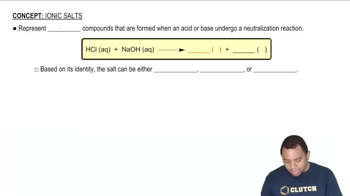

Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (b) NaBr
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (c) NaClO
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (d) 3CH3NH34NO3
Which member of each pair produces the more acidic aqueous solution: (a) ZnBr2 or CdCl2 (b) CuCl or Cu(NO3)2 (c) Ca(NO3)2 or NiBr2
An unknown salt is either NaF, NaCl, or NaOCl. When 0.050 mol of the salt is dissolved in water to form 0.500 L of solution, the pH of the solution is 8.08. What is the identity of the salt?