An unknown salt is either KBr, NH4Cl, KCN, or K2CO3. If a 0.100 M solution of the salt is neutral, what is the identity of the salt?
Ch.16 - Acid-Base Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 16, Problem 93
Predict the stronger acid in each pair: (a) HNO3 or HNO2 (b) H2SO4 or H2SeO4 (c) CH3COOH or CCl3COOH.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of acid strength. Acid strength is determined by the ability of an acid to donate a proton (H+). The more easily an acid donates a proton, the stronger it is.
Step 2: Analyze the molecular structure and electronegativity for each pair. For (a) HNO3 vs. HNO2, consider the number of oxygen atoms and their effect on the stability of the conjugate base. More oxygen atoms generally stabilize the conjugate base through resonance, making the acid stronger.
Step 3: For (b) H2SO4 vs. H2SeO4, compare the central atoms (S vs. Se). Sulfur is more electronegative than selenium, which can lead to a stronger acid due to better stabilization of the conjugate base.
Step 4: For (c) CH3COOH vs. CCl3COOH, examine the effect of substituents on the carboxylic acid group. The presence of electronegative chlorine atoms in CCl3COOH increases the acid strength by stabilizing the conjugate base through inductive effects.
Step 5: Conclude by identifying the stronger acid in each pair based on the analysis of molecular structure, electronegativity, and resonance or inductive effects.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Acid Strength and Ionization
The strength of an acid is determined by its ability to donate protons (H+) in solution. Strong acids completely ionize in water, releasing all their protons, while weak acids only partially ionize. The extent of ionization is influenced by the stability of the resulting conjugate base; more stable conjugate bases correspond to stronger acids.
Recommended video:
Guided course
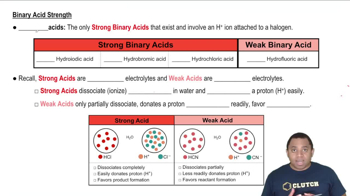
Binary Acid Strengths
Electronegativity and Inductive Effect
Electronegativity refers to the tendency of an atom to attract electrons. In acids, electronegative atoms can stabilize the negative charge of the conjugate base through the inductive effect, which enhances acid strength. For example, in halogenated acids, the presence of electronegative halogens can increase acidity by stabilizing the conjugate base.
Recommended video:
Guided course

Electronegativity Trends
Comparative Acid Strength in Oxoacids
In oxoacids, the number of oxygen atoms bonded to the central atom significantly affects acidity. More oxygen atoms generally lead to greater acid strength due to increased resonance stabilization of the conjugate base. Thus, when comparing oxoacids, the one with more oxygen atoms typically exhibits stronger acidic properties.
Recommended video:
Guided course
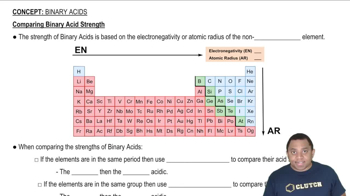
Comparing Binary Acid Strength
Related Practice
Textbook Question
Textbook Question
Which member of each pair produces the more acidic aqueous solution: (a) ZnBr2 or CdCl2 (b) CuCl or Cu(NO3)2 (c) Ca(NO3)2 or NiBr2
Textbook Question
An unknown salt is either NaF, NaCl, or NaOCl. When 0.050 mol of the salt is dissolved in water to form 0.500 L of solution, the pH of the solution is 8.08. What is the identity of the salt?
Textbook Question
Predict the stronger acid in each pair: (c) HBrO3 or HBrO2
Textbook Question
Predict the stronger acid in each pair: (e) benzoic acid (C6H5COOH) or phenol (C6H5OH).
Textbook Question
Based on their compositions and structures and on conjugate acid–base relationships, select the stronger base in each of the following pairs: (b) BrO- or BrO2-
