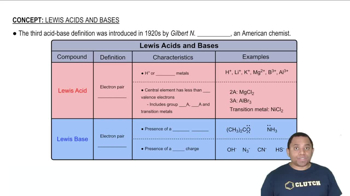
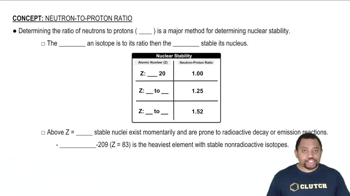
Textbook Question
The indicator methyl orange has been added to both of the following solutions. Based on the colors, classify each statement as true or false: (a) The pH of solution A is definitely less than 7.00. (b) The pH of solution B is definitely greater than 7.00. (c) The pH of solution B is greater than that of solution A.
1
views