Butyric acid is responsible for the foul smell of rancid butter. The pKa of butyric acid is 4.84. (a) Calculate the pKb for the butyrate ion.

Arrange the following 0.10 M solutions in order of increasing acidity: (i) NH4NO3, (ii) NaNO3, (iii) CH3COONH4, (iv) NaF, (v) CH3COONa.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
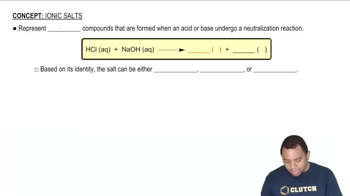
Acid-Base Theory

Salt Hydrolysis
pH and Acidity
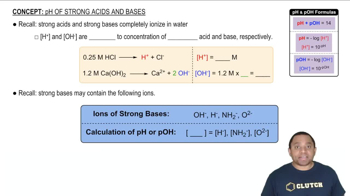
Butyric acid is responsible for the foul smell of rancid butter. The pKa of butyric acid is 4.84. (c) Calculate the pH of a 0.050 M solution of sodium butyrate.
The following observations are made about a diprotic acid H2A: (i) A 0.10 M solution of H2A has pH = 3.30. (ii) A 0.10 M solution of the salt NaHA is acidic. Which of the following could be the value of pKa2 for H2A: (i) 3.22, (ii) 5.30, (iii) 7.47, or (iv) 9.82?
The amino acid glycine (H2N–CH2–COOH) can participate in the following equilibria in water:
H2N–CH2–COOH + H2O ⇌ H2N–CH2–COO– + H3O+ Ka = 4.3 × 10-3
H2N–CH2–COOH + H2O⇌ +H3N–CH2–COOH + OH- Kb = 6.0 × 10-5
(a) Use the values of Ka and Kb to estimate the equilibrium constant for the intramolecular proton transfer to form a zwitterion: H2N–CH2–COOH ⇌ +H3N–CH2–COO–
