The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol, and the volume of the box is 1.0 L. (d) Assuming that all of the molecules are in the gas phase, calculate n, the change in the number of gas molecules that accompanies the reaction. [Section 15.2]

When lead(IV) oxide is heated above 300°C, it decomposes according to the reaction, 2 PbO2(𝑠)⇌2PbO(𝑠)+O2(𝑔). Consider the two sealed vessels of PbO2 shown here. If both vessels are heated to 400°C and allowed to come to equilibrium, which of the following statements is or are true?
b. The solid left at the bottom of each vessel will be a mixture of PbO2(𝑠) and PbO(𝑠).
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Chemical Equilibrium

Le Chatelier's Principle

Decomposition Reactions
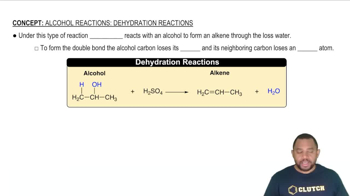
Ethene (C2H4) reacts with halogens (X2) by the following reaction:
C2H4(𝑔) + X2(𝑔) ⇌ C2H4X2(𝑔)
The following figures represent the concentrations at equilibrium at the same temperature when X2 is Cl2 (green), Br2 (brown), and I2 (purple). List the equilibria from smallest to largest equilibrium constant. [Section 15.3]
When lead(IV) oxide is heated above 300°C, it decomposes according to the reaction, 2 PbO2(𝑠) ⇌ 2PbO(𝑠) + O2(𝑔). Consider the two sealed vessels of PbO2 shown here. If both vessels are heated to 400°C and allowed to come to equilibrium, which of the following statements is or are true? a. There will be less PbO2 remaining in vessel A than in vessel B.
When lead(IV) oxide is heated above 300°C, it decomposes according to the reaction, 2 PbO2(𝑠)⇌2PbO(𝑠)+O2(𝑔). Consider the two sealed vessels of PbO2 shown here. If both vessels are heated to 400°C and allowed to come to equilibrium, which of the following statements is or are true? c. The partial pressure of O2(𝑔) will be the same in vessels A and B. [Section 15.4]
The reaction A2 + B2 ⇌ 2 AB has an equilibrium constant Kc = 1.5. The following diagrams represent reaction mixtures containing A2 molecules (red), B2 molecules (blue), and AB molecules. (a) Which reaction mixture is at equilibrium?
The diagram shown here represents the equilibrium state for the reaction A2(𝑔) + 2B(𝑔) ⇌ 2AB(𝑔). (a) Assuming the volume is 2 L, calculate the equilibrium constant 𝐾𝑐 for the reaction.
