The equilibrium 2 NO(𝑔) + Cl2(𝑔) ⇌ 2 NOCl(𝑔) is established at 500.0 K. An equilibrium mixture of the three gases has partial pressures of 0.095 atm, 0.171 atm, and 0.28 atm for NO, Cl2, and NOCl, respectively. (a) Calculate 𝐾𝑝 for this reaction at 500.0 K.
Ch.15 - Chemical Equilibrium

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 15, Problem 34a
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas: PCl3(𝑔) + Cl2(𝑔) ⇌ PCl5(𝑔). A 7.5-L gas vessel is charged with a mixture of PCl3(𝑔) and Cl2(𝑔), which is allowed to equilibrate at 450 K. At equilibrium the partial pressures of the three gases are 𝑃PCl3 = 0.124 atm, 𝑃Cl2 = 0.157 atm, and 𝑃PCl5 = 1.30 atm. (a) What is the value of 𝐾𝑝 at this temperature?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the problem. We are given the equilibrium partial pressures of PCl3, Cl2, and PCl5. We are asked to find the equilibrium constant, Kp, for the reaction at this temperature.
Step 2: Write down the expression for Kp. For the reaction aA + bB ⇌ cC + dD, the equilibrium constant expression is Kp = (PCc * PDd) / (PAa * PBb), where PX is the partial pressure of X.
Step 3: Substitute the given values into the Kp expression. For the reaction PCl3(𝑔) + Cl2(𝑔) ⇌ PCl5(𝑔), the Kp expression is Kp = PPCl5 / (PPCl3 * PCl2).
Step 4: Substitute the given partial pressures into the Kp expression. PPCl5 = 1.30 atm, PPCl3 = 0.124 atm, and PCl2 = 0.157 atm.
Step 5: Calculate the value of Kp by dividing the partial pressure of PCl5 by the product of the partial pressures of PCl3 and Cl2.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Equilibrium Constant (Kp)
The equilibrium constant (Kp) is a numerical value that expresses the ratio of the partial pressures of products to the partial pressures of reactants at equilibrium for a given reaction at a specific temperature. For the reaction PCl3(g) + Cl2(g) ⇌ PCl5(g), Kp is calculated using the formula Kp = (P_PCl5) / (P_PCl3 * P_Cl2), where P represents the partial pressures of the gases involved.
Recommended video:
Guided course

Equilibrium Constant Expressions
Partial Pressure
Partial pressure is the pressure exerted by a single component of a gas mixture. According to Dalton's Law of Partial Pressures, the total pressure of a gas mixture is the sum of the partial pressures of each individual gas. In this question, the partial pressures of PCl3, Cl2, and PCl5 are given, which are essential for calculating the equilibrium constant Kp.
Recommended video:
Guided course
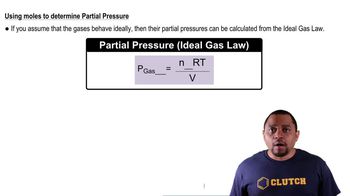
Partial Pressure Calculation
Le Chatelier's Principle
Le Chatelier's Principle states that if a dynamic equilibrium is disturbed by changing the conditions, the system will adjust itself to counteract the change and restore a new equilibrium. This principle helps in understanding how changes in concentration, pressure, or temperature can affect the position of equilibrium in reactions like the one involving PCl3, Cl2, and PCl5.
Recommended video:
Guided course

Le Chatelier's Principle
Related Practice
Textbook Question
Textbook Question
The equilibrium 2 NO(𝑔) + Cl2(𝑔) ⇌ 2 NOCl(𝑔) is established at 500.0 K. An equilibrium mixture of the three gases has partial pressures of 0.095 atm, 0.171 atm, and 0.28 atm for NO, Cl2, and NOCl, respectively. (b) If the vessel has a volume of 5.00 L, calculate Kc at this temperature.
Textbook Question
A mixture of 0.2000 mol of CO2, 0.1000 mol of H2, and 0.1600 mol of H2O is placed in a 2.000-L vessel. The following equilibrium is established at 500 K: CO2(g) + H2(g) ⇌ CO(g) + H2O(g) (d) Calculate Kc for the reaction.
1
views
