At 373 K, 𝐾𝑝 = 0.416 for the equilibrium 2 NOBr(𝑔) ⇌ 2 NO(𝑔) + Br2(𝑔) If the equilibrium partial pressures of NOBr(𝑔) and Br2(𝑔) are both 0.100 atm at 373 K, what is the equilibrium partial pressure of NO(𝑔)?

Consider the following equilibrium, for which Δ𝐻<0
2 SO2(𝑔) + O2(𝑔) ⇌ 2 SO3(𝑔)
(f) How will each of the following changes affect an equilibrium mixture of the three gases: SO3(𝑔) is removed from the system?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
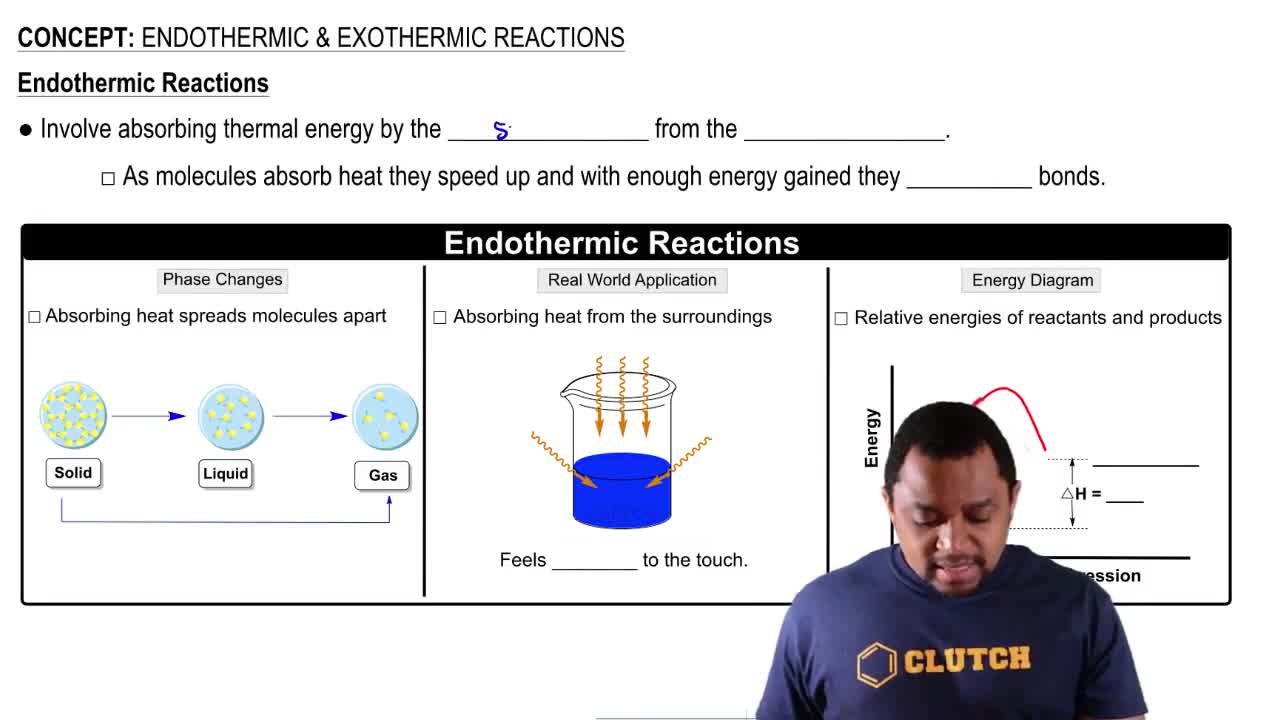
Key Concepts
Le Chatelier's Principle

Equilibrium Constant (K)

Endothermic vs. Exothermic Reactions
Consider the following equilibrium, for which Δ𝐻<0
2 SO2(𝑔) + O2(𝑔) ⇌ 2 SO3(𝑔)
(e) the total pressure of the system is increased by adding a noble gas
Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (a) increase [NH3] (b) increase [H2O] (c) decrease [O2]
Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (d) decrease the volume of the container in which the reaction occurs
Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (e) add a catalyst
