(b) If the temperature is raised by 100 K, does the equilibrium constant for this reaction increase or decrease?
Ch.15 - Chemical Equilibrium

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 15, Problem 73
A mixture of CH4 and H2O is passed over a nickel catalyst at 1000 K. The emerging gas is collected in a 5.00-L flask and is found to contain 8.62 g of CO, 2.60 g of H2, 43.0 g of CH4, and 48.4 g of H2O. Assuming that equilibrium has been reached, calculate Kc and Kp for the reaction CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g).
 Verified step by step guidance
Verified step by step guidance1
Step 1: Calculate the number of moles of each gas using their given masses and molar masses. Use the formula: \( n = \frac{\text{mass}}{\text{molar mass}} \).
Step 2: Determine the equilibrium concentrations of each gas by dividing the number of moles by the volume of the flask (5.00 L).
Step 3: Write the expression for the equilibrium constant \( K_c \) for the reaction: \( K_c = \frac{[\text{CO}][\text{H}_2]^3}{[\text{CH}_4][\text{H}_2O]} \). Substitute the equilibrium concentrations into this expression.
Step 4: Use the relationship between \( K_c \) and \( K_p \) to find \( K_p \). The formula is \( K_p = K_c(RT)^{\Delta n} \), where \( \Delta n \) is the change in moles of gas (products - reactants), \( R \) is the ideal gas constant (0.0821 L·atm/mol·K), and \( T \) is the temperature in Kelvin.
Step 5: Calculate \( \Delta n \) for the reaction, which is the difference in moles of gaseous products and reactants. Substitute \( \Delta n \), \( R \), and \( T \) into the \( K_p \) formula to find \( K_p \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Equilibrium Constant (Kc and Kp)
The equilibrium constant (Kc) is a ratio of the concentrations of products to reactants at equilibrium, raised to the power of their coefficients in the balanced equation. Kp is similar but uses partial pressures instead of concentrations. For the reaction CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g), Kc and Kp can be calculated using the molar concentrations or partial pressures of the gases involved, reflecting the extent of the reaction at equilibrium.
Recommended video:
Guided course

Kp vs. Kc Formula
Stoichiometry
Stoichiometry involves the quantitative relationships between the amounts of reactants and products in a chemical reaction. In this case, the stoichiometric coefficients from the balanced equation indicate that one mole of CH4 reacts with one mole of H2O to produce one mole of CO and three moles of H2. Understanding stoichiometry is essential for calculating the concentrations or partial pressures needed to determine Kc and Kp.
Recommended video:
Guided course

Stoichiometry Concept
Gas Laws and Molar Volume
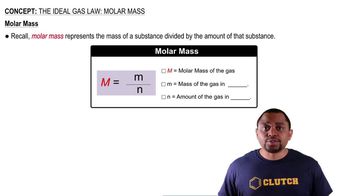
Gas laws describe the behavior of gases under various conditions of temperature and pressure. The ideal gas law (PV=nRT) relates pressure (P), volume (V), number of moles (n), the ideal gas constant (R), and temperature (T). In this problem, the volume of the gas collected (5.00 L) and the mass of each component allow for the calculation of moles, which are necessary for determining concentrations or partial pressures for the equilibrium constant calculations.
Recommended video:
Guided course
The Ideal Gas Law: Molar Mass
Related Practice
Textbook Question
Textbook Question
(c) If the temperature is raised by 100 K, does the forward rate constant kf increase by a larger or smaller amount than the reverse rate constant kr?
Textbook Question
When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (a) Calculate Kc for this reaction at this temperature.
1
views
Textbook Question
When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (c) According to Le Châtelier's principle, would the percent of SO2Cl2 that decomposes increase, decrease or stay the same if the mixture were transferred to a 15.00-L vessel?
1
views
