The gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea = 6.3 kJ>mol. and a frequency factor of A = 6.0 * 108 M-1 s-1. The reaction is believed to be bimolecular: NO1g2 + F21g2 ¡ NOF1g2 + F1g2 (e) Suggest a reason for the low activation energy for the reaction.
Ch.14 - Chemical Kinetics

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 14, Problem 122b
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride and hydrogen chloride:
Reaction 1: CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
This reaction is very slow in the absence of light. However, Cl2(g) can absorb light to form Cl atoms:
Reaction 2: Cl2(g) + hv → 2 Cl(g)
Once the Cl atoms are generated, they can catalyze the reaction of CH4 and Cl2, according to the following proposed mechanism:
Reaction 3: CH4(g) + Cl(g) → CH3(g) + HCl(g)
Reaction 4: CH3(g) + Cl2(g) → CH3Cl(g) + Cl(g)
The enthalpy changes and activation energies for these two reactions are tabulated as follows:
Reaction ΔH° (kJ/mol) Ea (kJ/mol)
3 +4 17
4 -109 4
(b) By using the data tabulated here, sketch a quantitative energy profile for the catalyzed reaction represented by reactions 3 and 4.

 Verified step by step guidance
Verified step by step guidance1
Identify the enthalpy changes (ΔH) and activation energies (Ea) for reactions 3 and 4 from the table provided.
For Reaction 3: Note that ΔH = +4 kJ/mol and Ea = 17 kJ/mol.
For Reaction 4: Note that ΔH = -109 kJ/mol and Ea = 4 kJ/mol.
Sketch the energy profile diagram: Start with the energy level of the reactants (CH4 and Cl2).
Draw the energy peaks for each reaction step, indicating the activation energies and the enthalpy changes, and finally show the energy level of the products (CH3Cl and HCl).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Activation Energy (Ea)
Activation energy is the minimum energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to form products. In the context of the given reactions, lower activation energy indicates that the reaction can proceed more quickly, which is crucial for understanding how light absorption by Cl2 facilitates the reaction with methane.
Recommended video:
Guided course

Activity Series Chart
Enthalpy Change (ΔH)
Enthalpy change refers to the heat content change during a chemical reaction at constant pressure. A negative ΔH indicates that the reaction is exothermic, releasing energy. In the provided reactions, the enthalpy changes help determine the overall energy profile and stability of the products compared to the reactants, which is essential for sketching the energy diagram.
Recommended video:
Guided course

Enthalpy of Formation
Catalysis
Catalysis is the process by which a substance (the catalyst) increases the rate of a chemical reaction without being consumed in the process. In this case, the Cl atoms generated from the light-absorbing Cl2 act as a catalyst, facilitating the reaction between methane and chlorine. Understanding catalysis is key to analyzing how the reaction mechanism is altered and how energy profiles are affected.
Recommended video:
Guided course
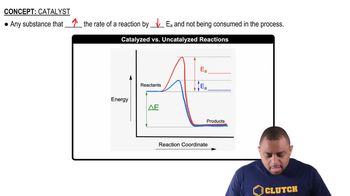
Catalyzed vs. Uncatalyzed Reactions
Related Practice
Textbook Question
Textbook Question
The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (a) Calculate the overall standard enthalpy change for the reaction process.
Textbook Question
The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (c) Draw a plausible Lewis structure for the intermediate HOOBr. To what familiar compound of hydrogen and oxygen does it appear similar?
Textbook Question
Many primary amines, RNH2, where R is a carboncontaining fragment such as CH3, CH3CH2, and so on, undergo reactions where the transition state is tetrahedral. (a) Draw a hybrid orbital picture to visualize the bonding at the nitrogen in a primary amine (just use a C atom for 'R').
