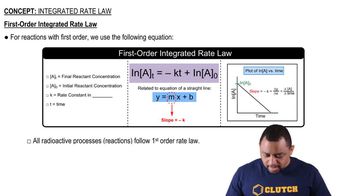
Americium-241 is used in smoke detectors. It has a first-order rate constant for radioactive decay of k = 1.6 * 10-3 yr-1. By contrast, iodine-125, which is used to test for thyroid functioning, has a rate constant for radioactive decay of k = 0.011 day-1. (a) What are the half-lives of these two isotopes? (b) Which one decays at a faster rate?

The rate of a first-order reaction is followed by spectroscopy, monitoring the absorbance of a colored reactant at 520 nm. The reaction occurs in a 1.00-cm sample cell, and the only colored species in the reaction has an extinction coefficient of 5.60 × 103 M-1 cm-1 at 520 nm.
(a) Calculate the initial concentration of the colored reactant if the absorbance is 0.605 at the beginning of the reaction.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Beer-Lambert Law
First-Order Reaction Kinetics
Extinction Coefficient
Americium-241 is used in smoke detectors. It has a first-order rate constant for radioactive decay of k = 1.6 * 10-3 yr-1. By contrast, iodine-125, which is used to test for thyroid functioning, has a rate constant for radioactive decay of k = 0.011 day-1. (c) How much of a 1.00-mg sample of each isotope remains after three half-lives? (d) How much of a 1.00-mg sample of each isotope remains after 4 days?
The rate of a first-order reaction is followed by spectroscopy, monitoring the absorbance of a colored reactant at 520 nm. The reaction occurs in a 1.00-cm sample cell, and the only colored species in the reaction has an extinction coefficient of 5.60 × 103 M-1 cm-1 at 520 nm.
(c) Calculate the half-life of the reaction.
The rate of a first-order reaction is followed by spectroscopy, monitoring the absorbance of a colored reactant at 520 nm. The reaction occurs in a 1.00-cm sample cell, and the only colored species in the reaction has an extinction coefficient of 5.60 × 103 M-1 cm-1 at 520 nm.
(d) How long does it take for the absorbance to fall to 0.100?
