Textbook Question
(a) What is a catalyst? (b) What is the difference between a homogeneous and a heterogeneous catalyst?

 Verified step by step guidance
Verified step by step guidance
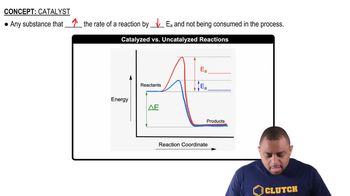
(a) What is a catalyst? (b) What is the difference between a homogeneous and a heterogeneous catalyst?
(c) Do catalysts affect the overall enthalpy change for a reaction, the activation energy, or both?
(a) Most commercial heterogeneous catalysts are extremely finely divided solid materials. Why is particle size important?
The addition of NO accelerates the decomposition of N2O, possibly by the following mechanism: NO1g2 + N2O1g2¡N21g2 + NO21g2 2 NO21g2¡2 NO1g2 + O21g2 (b) Is NO serving as a catalyst or an intermediate in this reaction?