Textbook Question
The reaction 2 NO2 → 2 NO + O2 has the rate constant k = 0.63 M-1s-1. (a) Based on the units for k, is the reaction first or second order in NO2?

 Verified step by step guidance
Verified step by step guidance
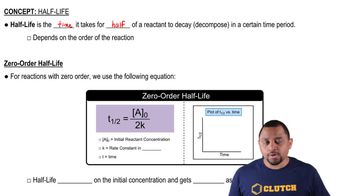
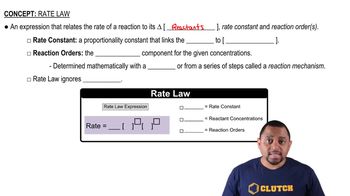

The reaction 2 NO2 → 2 NO + O2 has the rate constant k = 0.63 M-1s-1. (a) Based on the units for k, is the reaction first or second order in NO2?
The reaction 2 NO2 → 2 NO + O2 has the rate constant k = 0.63 M-1s-1.
(b) If the initial concentration of NO2 is 0.100 M, how would you determine how long it would take for the concentration to decrease to 0.025 M?
Americium-241 is used in smoke detectors. It has a first-order rate constant for radioactive decay of k = 1.6 * 10-3 yr-1. By contrast, iodine-125, which is used to test for thyroid functioning, has a rate constant for radioactive decay of k = 0.011 day-1. (a) What are the half-lives of these two isotopes? (b) Which one decays at a faster rate?