Textbook Question
What is the molecularity of each of the following elementary reactions? Write the rate law for each. (a) Cl2(g) → 2 Cl(g)

 Verified step by step guidance
Verified step by step guidance
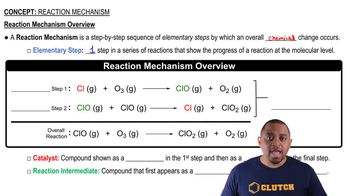
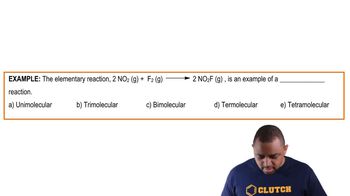

What is the molecularity of each of the following elementary reactions? Write the rate law for each. (a) Cl2(g) → 2 Cl(g)
The temperature dependence of the rate constant for a reaction is tabulated as follows: Temperature (K) k 1M 1 s1 2 600 0.028 650 0.22 700 1.3 750 6.0 800 23 Calculate Ea and A.
What is the molecularity of each of the following elementary reactions? Write the rate law for each. (b) OCl-(aq + H2O(l) → HOCl(aq) + OH-(aq)