Lauryl alcohol is obtained from coconut oil and is used to make detergents. A solution of 5.00 g of lauryl alcohol in 0.100 kg of benzene freezes at 4.1 °C. What is the molar mass of lauryl alcohol from these data? See Table 13.3 for the normal freezing point and 𝐾f of benzene.

What is the osmotic pressure formed by dissolving 44.2 mg of aspirin (C9H8O4) in 0.358 L of water at 25 °C?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Osmotic Pressure
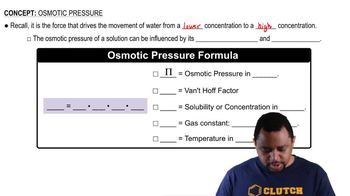
Molarity

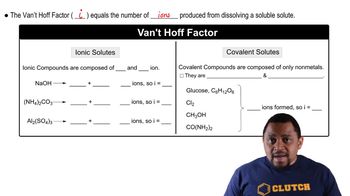
Van 't Hoff Factor
Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (a) 0.25 m glucose in ethanol; (b) 20.0 g of decane, C10H22, in 50.0 g CHCl3; (c) 3.50 g NaOH in 175 g of water, (d) 0.45 mol ethylene glycol and 0.15 mol KBr in 150 g H2O.
What is the freezing point of an aqueous solution that boils at 105.0 °C?
Seawater contains 34 g of salts for every liter of solution. Assuming that the solute consists entirely of NaCl (in fact, over 90% of the salt is indeed NaCl), calculate the osmotic pressure of seawater at 20 °C
Adrenaline is the hormone that triggers the release of extra glucose molecules in times of stress or emergency. A solution of 0.64 g of adrenaline in 36.0 g of CCl4 elevates the boiling point by 0.49 °C. Calculate the approximate molar mass of adrenaline from this data.
