Fluorocarbons (compounds that contain both carbon and fluorine) were, until recently, used as refrigerants. The compounds listed in the following table are all gases at 25 °C, and their solubilities in water at 25 °C and 1 atm fluorocarbon pressure are given as mass percentages. (c) Infants born with severe respiratory problems are sometimes given liquid ventilation: They breathe a liquid that can dissolve more oxygen than air can hold. One of these liquids is a fluorinated compound, CF3(CF2)7Br. The solubility of oxygen in this liquid is 66 mL O2 per 100 mL liquid. In contrast, air is 21% oxygen by volume. Calculate the moles of O2 present in an infant’s lungs (volume: 15 mL) if the infant takes a full breath of air compared to taking a full “breath” of a saturated solution of O2 in the fluorinated liquid. Assume a pressure of 1 atm in the lungs.
Ch.13 - Properties of Solutions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 13, Problem 110
The following table presents the solubilities of several gases in water at 25 °C under a total pressure of gas and water vapor of 1 atm. (b) The solubilities (in water) of the hydrocarbons are as follows: methane 6, ethane 6, and ethylene. Is this because ethylene is the most polar molecule? Gas Solubility (mM) CH4 (methane) 1.3 C2H6 (ethane) 1.8 C2H4 (ethylene) 4.7 N2 0.6 O2 1.2 NO 1.9 H2S 99 SO2 1476 (c) What intermolecular interactions can these hydrocarbons have with water? Gas Solubility (mM) CH4 (methane) 1.3 C2H6 (ethane) 1.8 C2H4 (ethylene) 4.7 N2 0.6 O2 1.2 NO 1.9 H2S 99 SO2 1476 (e) Explain why NO is more soluble in water than either N2 or O2. Gas Solubility (mM) CH4 (methane) 1.3 C2H6 (ethane) 1.8 C2H4 (ethylene) 4.7 N2 0.6 O2 1.2 NO 1.9 H2S 99 SO2 1476 (f) H2S is more water-soluble than almost all the other gases in the table. What intermolecular forces is H2S likely to have with water? Gas Solubility (mM) CH4 (methane) 1.3 C2H6 (ethane) 1.8 C2H4 (ethylene) 4.7 N2 0.6 O2 1.2 NO 1.9 H2S 99 SO2 1476 (g) SO2 is by far the most water-soluble gas in the table. What intermolecular forces is SO2 likely to have with water? Gas Solubility (mM) CH4 (methane) 1.3 C2H6 (ethane) 1.8 C2H4 (ethylene) 4.7 N2 0.6 O2 1.2 NO 1.9 H2S 99 SO2 1476
 Verified step by step guidance
Verified step by step guidance1
Step 1: Analyze the solubility data for hydrocarbons (methane, ethane, ethylene) and consider their molecular structures. Note that ethylene (C2H4) has a higher solubility than methane (CH4) and ethane (C2H6).
Step 2: Consider the polarity of the hydrocarbons. Ethylene has a double bond which can introduce some degree of polarity compared to the single bonds in methane and ethane, but overall, hydrocarbons are generally nonpolar.
Step 3: Examine the intermolecular interactions between hydrocarbons and water. Hydrocarbons are nonpolar and interact with water primarily through weak London dispersion forces, as water is polar and forms hydrogen bonds.
Step 4: Compare the solubility of NO, N2, and O2. Note that NO is more soluble than N2 and O2. Consider the molecular structure and polarity of NO, which has a polar bond due to the difference in electronegativity between nitrogen and oxygen.
Step 5: Investigate the intermolecular forces for H2S and SO2 with water. H2S can form hydrogen bonds with water due to its polar H-S bonds, while SO2, being a polar molecule with a bent shape, can engage in dipole-dipole interactions and possibly hydrogen bonding with water.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Polarity and Solubility
Polarity refers to the distribution of electrical charge over the atoms in a molecule. Polar molecules, which have a significant difference in electronegativity between their atoms, tend to dissolve well in polar solvents like water due to favorable interactions. In contrast, nonpolar molecules do not interact favorably with water, leading to lower solubility. Understanding the polarity of hydrocarbons like methane, ethane, and ethylene is crucial for explaining their solubility in water.
Recommended video:
Guided course

Molecular Polarity
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between molecules. These include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. The strength and type of these forces significantly influence the solubility of gases in water. For example, hydrogen sulfide (H2S) can form hydrogen bonds with water, enhancing its solubility compared to nonpolar gases like nitrogen (N2).
Recommended video:
Guided course

Intermolecular vs Intramolecular Forces
Henry's Law
Henry's Law states that the amount of gas that dissolves in a liquid at a given temperature is directly proportional to the partial pressure of that gas above the liquid. This principle helps explain the solubility of gases in water under varying conditions. For instance, the solubility of gases like nitric oxide (NO) can be understood in terms of its interactions with water and the pressure exerted by the gas, which influences how much of it can dissolve.
Recommended video:
Guided course
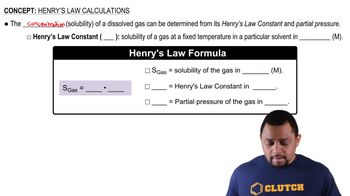
Henry's Law Calculations
Related Practice
Textbook Question
Textbook Question
At ordinary body temperature (37 °C), the solubility of N2 in water at ordinary atmospheric pressure (1.0 atm) is 0.015 g/L. Air is approximately 78 mol % N2. (b) At a depth of 100 ft in water, the external pressure is 4.0 atm. What is the solubility of N2 from air in blood at this pressure?
Textbook Question
Compounds like sodium stearate, called 'surfactants' in general, can form structures known as micelles in water, once the solution concentration reaches the value known as the critical micelle concentration (cmc). Micelles contain dozens to hundreds of molecules. The cmc depends on the substance, the solvent, and the temperature. (a) The turbidity (the amount of light scattering) of solutions increases dramatically at the cmc. Suggest an explanation. .
