For the dissolution of LiCl in water, ∆Hsoln = -37 kJ/mol. Which term would you expect to be the largest negative number: ∆Hsolvent, ∆Hsolute, or ∆Hmix?

KBr is relatively soluble in water, yet its enthalpy of solution is + 19.8 kJ/mol. Which of the following statements provides the best explanation for this behavior? (a) Potassium salts are always soluble in water. (b) The entropy of mixing must be unfavorable. (c) The enthalpy of mixing must be small compared to the enthalpies for breaking up water–water interactions and K–Br ionic interactions. (d) KBr has a high molar mass compared to other salts like NaCl.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
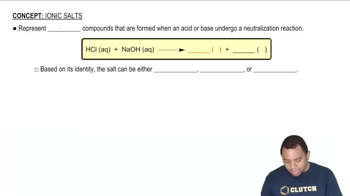
Key Concepts
Enthalpy of Solution

Entropy and Mixing

Ionic Interactions
Two nonpolar organic liquids, hexane (C6H14) and heptane (C7H16), are mixed. (a) Do you expect ∆Hsoln to be a large positive number, a large negative number, or close to zero? Explain.
Two nonpolar organic liquids, hexane (C6H14) and heptane (C7H16), are mixed. (b) Hexane and heptane are miscible with each other in all proportions. In making a solution of them, is the entropy of the system increased, decreased, or close to zero, compared to the separate pure liquids?
By referring to Figure 13.15, determine whether the addition of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution:(c) K2Cr2O7
By referring to Figure 13.15, determine whether the addition of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution: (d) Pb(NO3)2.
