Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (a) 0.22 m glycerol (C3H8O3) in ethanol, (b) 0.240 mol of naphthalene (C10H8) in 2.45 mol of chloroform, (c) 1.50 g NaCl in 0.250 kg of water, (d) 2.04 g KBr and 4.82 g of glucose (C6H12O6) in 188 g of water.
Ch.13 - Properties of Solutions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 13, Problem 77
How many grams of ethylene glycol (C2H6O2) must be added to 1.00 kg of water to produce a solution that freezes at -5.00 °C?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the freezing point depression formula: \( \Delta T_f = i \cdot K_f \cdot m \), where \( \Delta T_f \) is the change in freezing point, \( i \) is the van't Hoff factor, \( K_f \) is the freezing point depression constant for water, and \( m \) is the molality of the solution.
Step 2: Calculate \( \Delta T_f \) by subtracting the freezing point of the solution (-5.00 °C) from the normal freezing point of water (0.00 °C).
Step 3: Use the van't Hoff factor \( i = 1 \) for ethylene glycol, as it does not dissociate in solution, and find the value of \( K_f \) for water, which is typically 1.86 °C kg/mol.
Step 4: Rearrange the freezing point depression formula to solve for molality \( m \): \( m = \frac{\Delta T_f}{i \cdot K_f} \).
Step 5: Calculate the mass of ethylene glycol needed using the formula \( m = \frac{\text{moles of solute}}{\text{kg of solvent}} \), and convert moles to grams using the molar mass of ethylene glycol (C2H6O2), which is approximately 62.07 g/mol.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Freezing Point Depression
Freezing point depression is a colligative property that describes how the addition of a solute to a solvent lowers the freezing point of the solvent. The extent of this depression depends on the number of solute particles in the solution, not their identity. The formula used to calculate the change in freezing point is ΔTf = i * Kf * m, where i is the van 't Hoff factor, Kf is the freezing point depression constant of the solvent, and m is the molality of the solution.
Recommended video:
Guided course

Freezing Point Depression
Molality
Molality (m) is a measure of concentration defined as the number of moles of solute per kilogram of solvent. It is particularly useful in colligative property calculations because it directly relates to the number of solute particles in a given mass of solvent. To find molality, you can use the formula m = moles of solute / mass of solvent (in kg), which helps in determining how much solute is needed to achieve a desired freezing point.
Recommended video:
Guided course
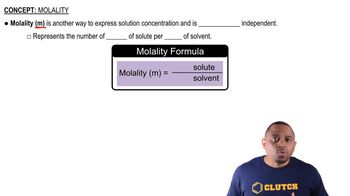
Molality
Ethylene Glycol Properties
Ethylene glycol (C2H6O2) is a common antifreeze agent that, when dissolved in water, significantly lowers the freezing point of the solution. Understanding its molecular weight (62.07 g/mol) is essential for converting between grams and moles, which is necessary for calculating how much ethylene glycol is needed to achieve the desired freezing point depression. Its van 't Hoff factor is 1, as it does not dissociate into ions in solution.
Recommended video:
Guided course
Physical Properties
Related Practice
Textbook Question
Textbook Question
Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (a) 0.25 m glucose in ethanol; (b) 20.0 g of decane, C10H22, in 50.0 g CHCl3; (c) 3.50 g NaOH in 175 g of water, (d) 0.45 mol ethylene glycol and 0.15 mol KBr in 150 g H2O.
1
views
Textbook Question
What is the freezing point of an aqueous solution that boils at 105.0 °C?
Textbook Question
Seawater contains 34 g of salts for every liter of solution. Assuming that the solute consists entirely of NaCl (in fact, over 90% of the salt is indeed NaCl), calculate the osmotic pressure of seawater at 20 °C
