Calculate the number of moles of solute present in each of the following solutions: (a) 255 mL of 1.50 M HNO3(aq),
Ch.13 - Properties of Solutions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 13, Problem 54a
Describe how you would prepare each of the following aqueous solutions from water and the solid solute: a. 1.50 L of 0.110 M (NH4)2SO4 solution;
 Verified step by step guidance
Verified step by step guidance1
Determine the molar mass of \((NH_4)_2SO_4\) by adding the atomic masses of all atoms in the formula.
Calculate the number of moles of \((NH_4)_2SO_4\) needed using the formula: \(\text{moles} = \text{molarity} \times \text{volume in liters}\).
Convert the moles of \((NH_4)_2SO_4\) to grams using the molar mass calculated in step 1.
Weigh the calculated mass of \((NH_4)_2SO_4\) using a balance.
Dissolve the weighed \((NH_4)_2SO_4\) in a volume of water less than 1.50 L, then dilute the solution to a final volume of 1.50 L in a volumetric flask.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molarity
Molarity (M) is a measure of concentration defined as the number of moles of solute per liter of solution. It is crucial for preparing solutions, as it allows chemists to quantify how much solute is needed to achieve a desired concentration in a given volume of solvent. For example, a 0.110 M solution of (NH4)2SO4 means there are 0.110 moles of ammonium sulfate in every liter of solution.
Recommended video:
Guided course

Molarity
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. Understanding dilution is essential when preparing solutions, as it helps in calculating the amount of solute needed to achieve a specific molarity. The dilution formula, M1V1 = M2V2, relates the initial and final concentrations and volumes, guiding the preparation process.
Recommended video:
Guided course
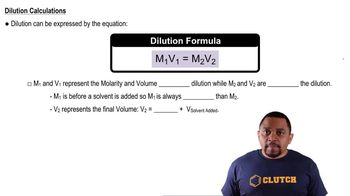
Dilution Equation
Solubility
Solubility refers to the maximum amount of solute that can dissolve in a solvent at a given temperature and pressure. It is important to know the solubility of (NH4)2SO4 when preparing the solution to ensure that the solute can fully dissolve in the solvent without forming a precipitate. This concept helps in determining whether the desired concentration can be achieved with the available solute.
Recommended video:
Guided course

Solubility Rules
Related Practice
Textbook Question
Textbook Question
Describe how you would prepare each of the following aqueous solutions from water and solid KBr: b. 125 g of 0.180 m KBr.
1
views
Textbook Question
Commercial aqueous nitric acid has a density of 1.42 g/mL and is 16 M. Calculate the percent HNO3 by mass in the solution.
Textbook Question
Brass is a substitutional alloy consisting of a solution of copper and zinc. A particular sample of red brass consisting of 80.0 % Cu and 20.0 % Zn by mass has a density of 8750 kg/m3. (a) What is the molality of Zn in the solid solution?
Textbook Question
Brass is a substitutional alloy consisting of a solution of copper and zinc. A particular sample of red brass consisting of 80.0 % Cu and 20.0 % Zn by mass has a density of 8750 kg/m3. (b) What is the molarity of Zn in the solution?
