What is the molarity of each of the following solutions: (b) 5.25 g of Mn(NO3)2⋅2H2O in 175 mL of solution,
Ch.13 - Properties of Solutions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 13, Problem 48a
Ascorbic acid (vitamin C, C6H8O6) is a water-soluble vitamin. A solution containing 80.5 g of ascorbic acid dissolved in 210 g of water has a density of 1.22 g/mL at 55 °C. Calculate (a) the mass percentage,
 Verified step by step guidance
Verified step by step guidance1
Identify the formula for mass percentage: \( \text{Mass percentage} = \left( \frac{\text{mass of solute}}{\text{mass of solution}} \right) \times 100 \% \).
Determine the mass of the solute, which is the ascorbic acid, given as 80.5 g.
Calculate the mass of the solution by adding the mass of the solute (ascorbic acid) to the mass of the solvent (water), which is 210 g.
Substitute the values into the mass percentage formula: \( \text{Mass percentage} = \left( \frac{80.5 \text{ g}}{\text{mass of solution}} \right) \times 100 \% \).
Simplify the expression to find the mass percentage of ascorbic acid in the solution.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Mass Percentage
Mass percentage is a way of expressing the concentration of a component in a mixture. It is calculated by taking the mass of the solute (in this case, ascorbic acid) and dividing it by the total mass of the solution (solute plus solvent), then multiplying by 100 to convert it to a percentage. This concept is essential for understanding how much of a substance is present in a solution relative to the total mass.
Recommended video:
Guided course

Mass Percent Calculation
Density
Density is defined as mass per unit volume and is a critical property of substances that can affect calculations in chemistry. In this context, the density of the solution (1.22 g/mL) can be used to determine the volume of the solution, which is necessary for calculating the mass percentage. Understanding density helps in converting between mass and volume, especially in solutions.
Recommended video:
Guided course
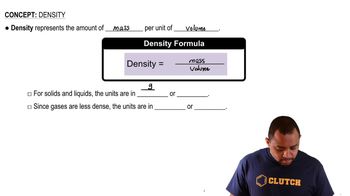
Density Concepts
Solution Composition
The composition of a solution refers to the relative amounts of solute and solvent present. In this case, ascorbic acid is the solute, and water is the solvent. Knowing the composition is vital for calculating various properties of the solution, including mass percentage and molarity, which are important for understanding the behavior of the solution in chemical reactions.
Recommended video:
Guided course
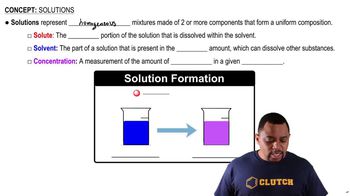
Solution Components
Related Practice
Textbook Question
2
views
Textbook Question
Ascorbic acid (vitamin C, C6H8O6) is a water-soluble vitamin. A solution containing 80.5 g of ascorbic acid dissolved in 210 g of water has a density of 1.22 g/mL at 55 °C. Calculate (c) the molality,
Textbook Question
The density of acetonitrile (CH3CN) is 0.786 g/mL and the density of methanol (CH3OH) is 0.791 g/mL. A solution is made by dissolving 22.5 mL of CH3OH in 98.7 mL of CH3CN. (a) What is the mole fraction of methanol in the solution?
Textbook Question
The density of acetonitrile (CH3CN) is 0.786 g/mL and the density of methanol (CH3OH) is 0.791 g/mL. A solution is made by dissolving 22.5 mL of CH3OH in 98.7 mL of CH3CN. (b) What is the molality of the solution?
