Fluorocarbons (compounds that contain both carbon and fluorine) were, until recently, used as refrigerants. The compounds listed in the following table are all gases at 25 °C, and their solubilities in water at 25 °C and 1 atm fluorocarbon pressure are given as mass percentages. (a) For each fluorocarbon, calculate the molality of a saturated solution.

A lithium salt used in lubricating grease has the formula LiC nH2n + 1O2. The salt is soluble in water to the extent of 0.036 g per 100 g of water at 25 °C. The osmotic pressure of this solution is found to be 57.1 torr. Assuming that molality and molarity in such a dilute solution are the same and that the lithium salt is completely dissociated in the solution, determine an appropriate value of n in the formula for the salt.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Osmotic Pressure
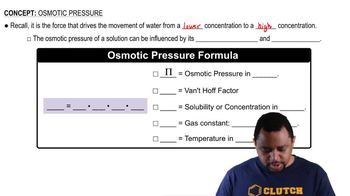
Dissociation of Ionic Compounds

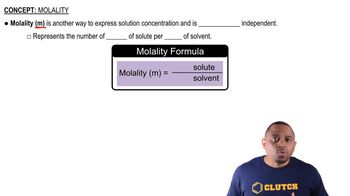
Molality and Molarity
Fluorocarbons (compounds that contain both carbon and fluorine) were, until recently, used as refrigerants. The compounds listed in the following table are all gases at 25 °C, and their solubilities in water at 25 °C and 1 atm fluorocarbon pressure are given as mass percentages. (b) Which molecular property best predicts the solubility of these gases in water: molar mass, dipole moment, or ability to hydrogen-bond to water?
Fluorocarbon Solubility (mass %)
CF4 0.0015
CClF3 0.009
CCl2F2 0.028
CHClF2 0.30
Fluorocarbons (compounds that contain both carbon and fluorine) were, until recently, used as refrigerants. The compounds listed in the following table are all gases at 25 °C, and their solubilities in water at 25 °C and 1 atm fluorocarbon pressure are given as mass percentages. (c) Infants born with severe respiratory problems are sometimes given liquid ventilation: They breathe a liquid that can dissolve more oxygen than air can hold. One of these liquids is a fluorinated compound, CF3(CF2)7Br. The solubility of oxygen in this liquid is 66 mL O2 per 100 mL liquid. In contrast, air is 21% oxygen by volume. Calculate the moles of O2 present in an infant’s lungs (volume: 15 mL) if the infant takes a full breath of air compared to taking a full “breath” of a saturated solution of O2 in the fluorinated liquid. Assume a pressure of 1 atm in the lungs.
At ordinary body temperature (37 °C), the solubility of N2 in water at ordinary atmospheric pressure (1.0 atm) is 0.015 g/L. Air is approximately 78 mol % N2. (b) At a depth of 100 ft in water, the external pressure is 4.0 atm. What is the solubility of N2 from air in blood at this pressure?
Compounds like sodium stearate, called 'surfactants' in general, can form structures known as micelles in water, once the solution concentration reaches the value known as the critical micelle concentration (cmc). Micelles contain dozens to hundreds of molecules. The cmc depends on the substance, the solvent, and the temperature. (a) The turbidity (the amount of light scattering) of solutions increases dramatically at the cmc. Suggest an explanation. .
