Silicon is the fundamental component of integrated circuits. Si has the same structure as diamond. (a) Is Si a molecular, metallic, ionic, or covalent-network solid?
Ch.12 - Solids and Modern Materials

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 12, Problem 13a
What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (a) molecular crystals?
 Verified step by step guidance
Verified step by step guidance1
Identify the type of particles present in molecular crystals: In molecular crystals, the particles are molecules.
Understand the nature of molecular interactions: Molecular crystals are held together primarily by intermolecular forces rather than chemical bonds.
Recognize the types of intermolecular forces: The main types of intermolecular forces include London dispersion forces, dipole-dipole interactions, and hydrogen bonds.
Analyze the strength and characteristics of these forces: London dispersion forces are generally weaker and occur due to temporary dipoles. Dipole-dipole interactions occur between molecules with permanent dipoles. Hydrogen bonds are a stronger type of dipole-dipole interaction that occurs specifically when hydrogen is bonded to a highly electronegative atom like oxygen, nitrogen, or fluorine.
Apply this understanding to molecular crystals: In molecular crystals, the type of intermolecular force present depends on the specific molecules that make up the crystal. For example, ice (a molecular crystal of water) exhibits hydrogen bonding due to the polar nature of water molecules.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Intermolecular Forces
Intermolecular forces are the attractive forces that occur between molecules. These forces include hydrogen bonding, dipole-dipole interactions, and London dispersion forces. They play a crucial role in determining the physical properties of substances, such as boiling and melting points, especially in molecular crystals where these forces dictate how molecules are arranged and interact.
Recommended video:
Guided course

Intermolecular vs Intramolecular Forces
Molecular Crystals
Molecular crystals are solid structures formed by molecules held together primarily by intermolecular forces rather than ionic or covalent bonds. These crystals typically have lower melting points compared to ionic or metallic crystals due to the weaker nature of the intermolecular forces. Understanding the arrangement and types of molecules in these crystals is essential for predicting their properties and behaviors.
Recommended video:
Guided course
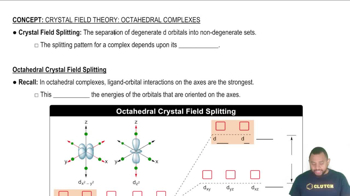
The crystal field splitting pattern for octahedral complexes has the d orbitals on or along the axes as having the higher energy.
Types of Attractive Forces
The types of attractive forces in molecular crystals include hydrogen bonds, which are strong interactions between molecules containing hydrogen bonded to electronegative atoms; dipole-dipole interactions, which occur between polar molecules; and London dispersion forces, which are weak attractions due to temporary dipoles in nonpolar molecules. Each type of force contributes differently to the stability and characteristics of the crystal structure.
Recommended video:
Guided course
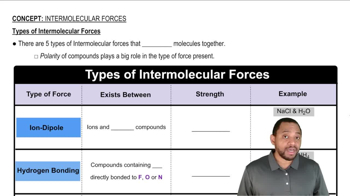
Types of Intermolecular Forces
Related Practice
Textbook Question
Textbook Question
Silicon is the fundamental component of integrated circuits. Si has the same structure as diamond. (b) Silicon readily reacts to form silicon dioxide, SiO2, which is quite hard and is insoluble in water. Is SiO2 most likely a molecular, metallic, ionic, or covalent-network solid?
Textbook Question
What kinds of attractive forces exist between particles (atoms, molecules, or ions) in (d) and metallic crystals?
Textbook Question
Which type (or types) of crystalline solid is characterized by each of the following? (a) High mobility of electrons throughout the solid;
Textbook Question
Which type (or types) of crystalline solid is characterized by each of the following? (b) softness, relatively low melting point;
