CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (b) Would appropriately sized CdS quantum dots be able to emit blue light?
Ch.12 - Solids and Modern Materials

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 12, Problem 93
If you want to make a polymer for plastic wrap, should you strive to make a polymer that has a high or low degree of crystallinity?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of crystallinity in polymers. Crystallinity refers to the degree of structural order in a polymer. A high degree of crystallinity means the polymer chains are well-organized and packed closely together, while a low degree of crystallinity means the chains are more disordered.
Step 2: Consider the properties of polymers with high crystallinity. These polymers tend to be more rigid, have higher melting points, and are less permeable to gases and moisture. This is due to the tight packing of the polymer chains.
Step 3: Consider the properties of polymers with low crystallinity. These polymers are generally more flexible, have lower melting points, and are more permeable to gases and moisture. The disordered arrangement of chains allows for more movement and flexibility.
Step 4: Relate these properties to the desired characteristics of plastic wrap. Plastic wrap should be flexible, able to stretch and conform to the shape of the items it covers, and provide a barrier to moisture and gases to preserve food.
Step 5: Conclude whether a high or low degree of crystallinity is more suitable for plastic wrap based on the desired properties. Consider that flexibility and barrier properties are key factors in determining the appropriate degree of crystallinity for this application.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Degree of Crystallinity
The degree of crystallinity refers to the extent to which a polymer's molecular chains are ordered in a regular, repeating pattern. High crystallinity typically results in materials that are more rigid and have higher melting points, while low crystallinity leads to more amorphous structures that are often more flexible and transparent. Understanding this concept is crucial for determining the physical properties of polymers used in applications like plastic wrap.
Recommended video:
Guided course
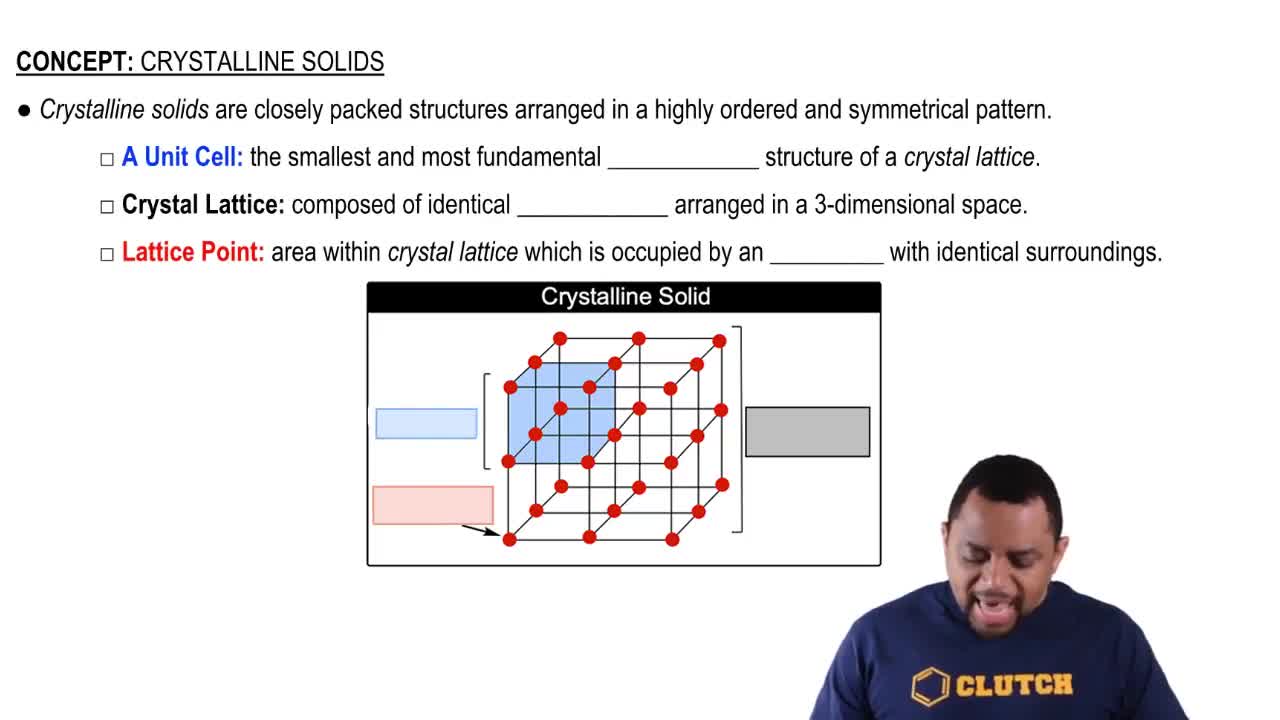
Crystalline Solids Structure
Polymer Properties
Polymers exhibit a range of properties based on their molecular structure, including tensile strength, flexibility, and transparency. For plastic wrap, a desirable property is flexibility, which allows the material to conform to various shapes. The balance between crystallinity and amorphous regions in a polymer influences these properties, making it essential to consider when designing materials for specific applications.
Recommended video:
Guided course
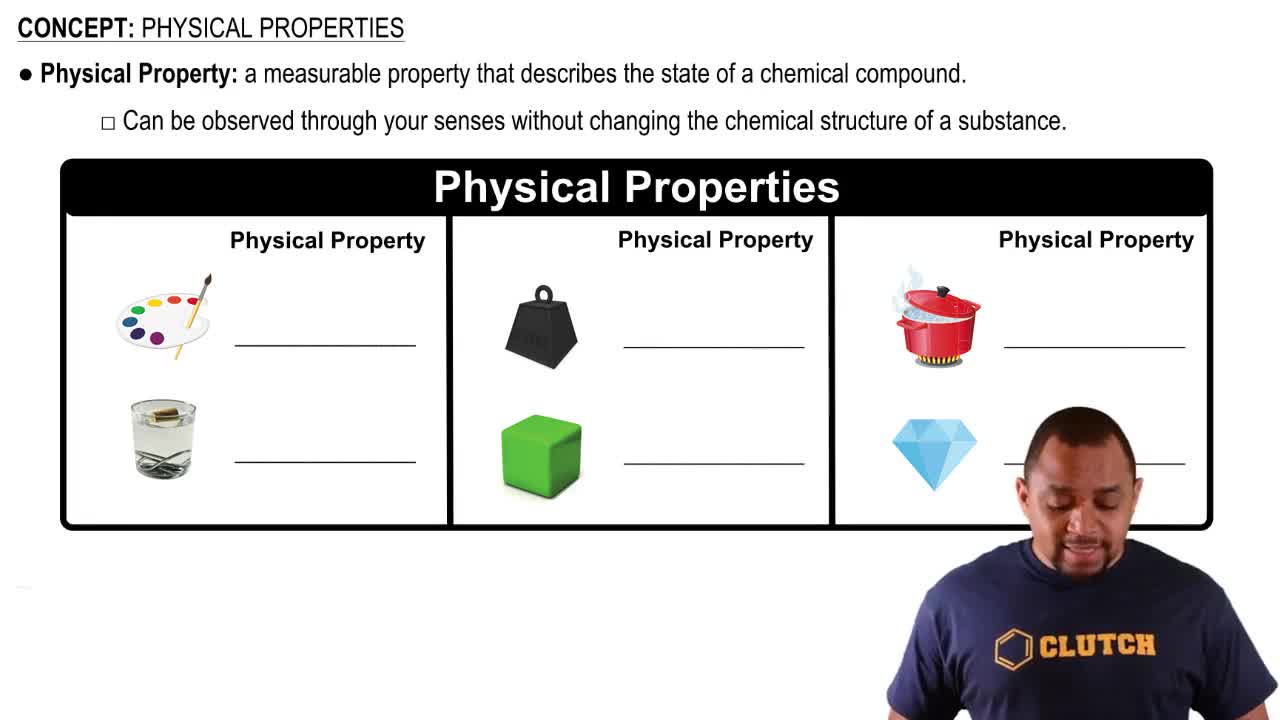
Physical Properties
Amorphous vs. Crystalline Polymers
Amorphous polymers lack a well-defined structure, resulting in materials that are generally more flexible and transparent, while crystalline polymers have a more ordered arrangement, leading to increased strength and rigidity. For plastic wrap, a lower degree of crystallinity is preferred to ensure that the material is flexible and can easily stretch around food items, providing a better seal and protection.
Recommended video:
Guided course
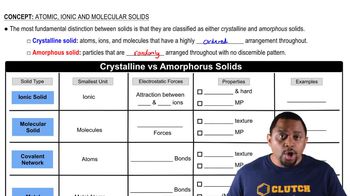
Crystalline vs Amorphous Solids
Related Practice
Textbook Question
Textbook Question
Write the chemical equation that represents the formation of (b) polyacrylonitrile from acrylonitrile (polyacrylonitrile is used in home furnishings, craft yarns, clothing, and many other items).
Textbook Question
What molecular structural features cause high-density polyethylene to be denser than low-density polyethylene?
Textbook Question
Indicate whether each statement is true or false: (a) Elastomers are rubbery solids. (b) Thermosets cannot be reshaped. (c) Thermoplastic polymers can be recycled.
Textbook Question
CdS has a band gap of 2.4 eV. If large crystals of CdS are illuminated with ultraviolet light, they emit light equal to the band gap energy. (a) What color is the emitted light?
