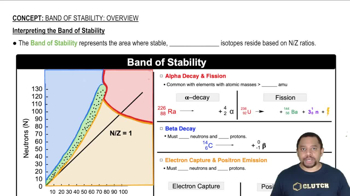
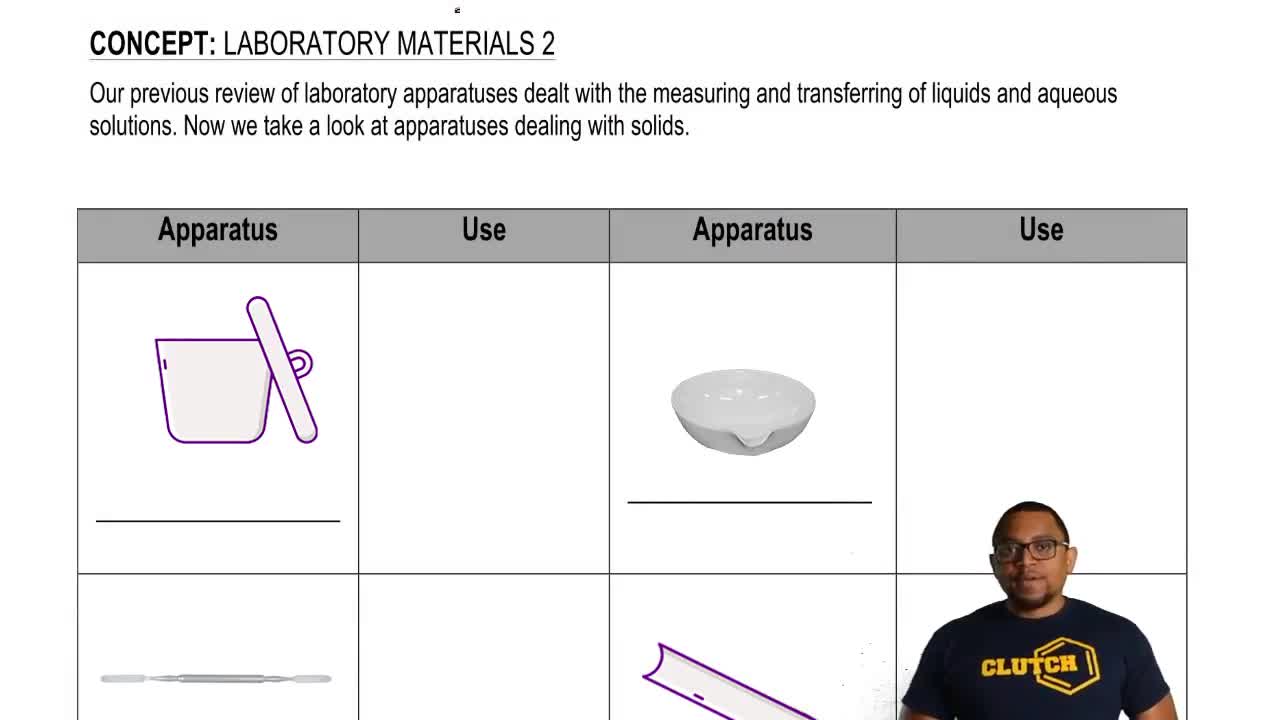
Textbook Question
Both covalent-network solids and ionic solids can have melting points well in excess of room temperature, and both can be poor conductors of electricity in their pure form. However, in other ways their properties are quite different. (a) Which type of solid is more likely to dissolve in water?