Which of these statements about alloys and intermetallic compounds is false? (a) Bronze is an example of an alloy. (b) 'Alloy' is just another word for 'a chemical compound of fixed composition that is made of two or more metals.' (c) Intermetallics are compounds of two or more metals that have a definite composition and are not considered alloys. (d) If you mix two metals together and, at the atomic level, they separate into two or more different compositional phases, you have created a heterogeneous alloy. (e) Alloys can be formed even if the atoms that comprise them are rather different in size.

For each of the following alloy compositions, indicate whether you would expect it to be a substitutional alloy, an interstitial alloy, or an intermetallic compound: (a) Cu₀.₆₆Zn₀.₃₄ (b) Ag₃Sn (c) Ti₀.₉₉O₀.₀₁.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Substitutional Alloys
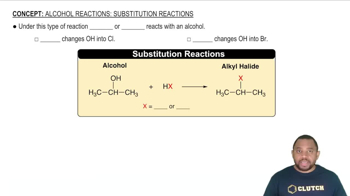
Interstitial Alloys
Intermetallic Compounds

Determine if each statement is true or false: (b) Substitutional alloys have 'solute' atoms that replace 'solvent' atoms in a lattice, but interstitial alloys have 'solute' atoms that are in between the 'solvent' atoms in a lattice.
For each of the following alloy compositions, indicate whether you would expect it to be a substitutional alloy, an interstitial alloy, or an intermetallic compound: (a) Fe0.97Si0.03 (b) Fe0.60Ni0.40 (c) SmCo5.
Indicate whether each statement is true or false: (c) Nonmetallic elements are never found in alloys.
An increase in temperature causes most metals to undergo thermal expansion, which means the volume of the metal increases upon heating. How does thermal expansion affect the unit cell length? What is the effect of an increase in temperature on the density of a metal?
