Sodium metal (atomic weight 22.99 g/mol) adopts a body-centered cubic structure with a density of 0.97 g/cm3. (a) Use this information and Avogadro’s number (NA = 6.022 × 1023/mol) to estimate the atomic radius of sodium. (b) If sodium didn't react so vigorously, it could float on water. Use the answer from part (a) to estimate the density of Na if its structure were that of a cubic close-packed metal. Would it still float on water?
Ch.12 - Solids and Modern Materials

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 12, Problem 36b,c,d
Calcium crystallizes in a body-centered cubic structure at 467°C. (b) How many nearest neighbors does each Ca atom possess? (c) Estimate the length of the unit cell edge, a, from the atomic radius of calcium (1.97 Å). (d) Estimate the density of Ca metal at this temperature.
 Verified step by step guidance
Verified step by step guidance1
To determine the number of nearest neighbors in a body-centered cubic (BCC) structure, note that each atom in a BCC lattice has 8 nearest neighbors. This is because the central atom in the cube is surrounded by 8 corner atoms.
To estimate the length of the unit cell edge (a) in a BCC structure, use the relationship between the atomic radius (r) and the edge length: \( a = \frac{4r}{\sqrt{3}} \). Substitute the given atomic radius of calcium (1.97 Å) into this formula to find the edge length.
To estimate the density of calcium metal, first calculate the volume of the unit cell using the edge length: \( V = a^3 \).
Next, determine the mass of the unit cell. In a BCC structure, there are 2 atoms per unit cell. Calculate the mass of these atoms using the molar mass of calcium (40.08 g/mol) and Avogadro's number (6.022 x 10^23 atoms/mol).
Finally, calculate the density using the formula \( \text{Density} = \frac{\text{Mass of unit cell}}{\text{Volume of unit cell}} \). Convert the mass to grams and the volume to cubic centimeters to find the density in g/cm³.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Body-Centered Cubic (BCC) Structure
A body-centered cubic structure is a type of crystal lattice where atoms are located at each corner of a cube and a single atom is positioned at the center of the cube. In this arrangement, each atom has eight nearest neighbors, which are the corner atoms. Understanding this structure is crucial for determining the coordination number and the geometric properties of the material.
Recommended video:
Guided course

Body Centered Cubic Example
Unit Cell and Atomic Radius
The unit cell is the smallest repeating unit in a crystal lattice that reflects the symmetry and structure of the entire crystal. For a BCC structure, the relationship between the atomic radius and the unit cell edge length can be derived from geometry, specifically using the formula a = 4r/√3, where 'a' is the edge length and 'r' is the atomic radius. This relationship allows for the calculation of the unit cell dimensions based on atomic size.
Recommended video:
Guided course
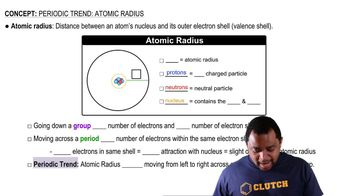
Atomic Radius
Density Calculation
Density is defined as mass per unit volume and can be calculated using the formula ρ = m/V, where 'm' is the mass of the substance and 'V' is the volume. In the context of a crystal structure, the mass can be determined from the molar mass and the number of atoms in the unit cell, while the volume is derived from the cube of the unit cell edge length. This concept is essential for estimating the density of materials based on their crystalline structure.
Recommended video:
Guided course
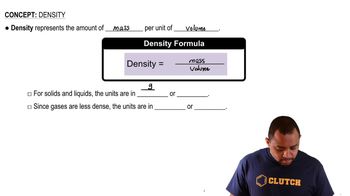
Density Concepts
Related Practice
Textbook Question
Textbook Question
Calcium crystallizes in a body-centered cubic structure at 467°C. (a) How many Ca atoms are contained in each unit cell?
Textbook Question
Calcium crystallizes in a face-centered cubic unit cell at room temperature that has an edge length of 5.588 Å.
a. Calculate the atomic radius of a calcium atom.
b. Calculate the density of Ca metal at this temperature.
