(b) What is the relationship between viscosity and temperature?
Ch.11 - Liquids and Intermolecular Forces

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 11, Problem 37b
Liquids can interact with flat surfaces just as they can with capillary tubes; the cohesive forces within the liquid can be stronger or weaker than the adhesive forces between liquid and surface:![]()
(b) Which of these diagrams, i or ii, rep- resents what happens when water is on a nonpolar surface?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concepts of cohesive and adhesive forces. Cohesive forces are the intermolecular forces between the molecules of the liquid, while adhesive forces are the forces between the liquid molecules and the surface.
Step 2: Recognize that water is a polar molecule, meaning it has a partial positive charge on one side and a partial negative charge on the other.
Step 3: Identify that a nonpolar surface does not have a charge distribution that can interact strongly with the polar water molecules.
Step 4: Compare the two diagrams (i and ii) to determine which one shows water on a nonpolar surface. Look for signs of weak interaction between the water and the surface, such as water forming droplets due to strong cohesive forces within the water.
Step 5: Conclude that the diagram where water forms droplets (indicating strong cohesive forces and weak adhesive forces) represents water on a nonpolar surface.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Cohesive Forces
Cohesive forces are the intermolecular forces that hold molecules of the same substance together. In liquids, these forces are responsible for phenomena such as surface tension, where the molecules at the surface experience a net inward force due to stronger interactions with neighboring molecules. Understanding cohesive forces is essential for predicting how a liquid behaves on different surfaces.
Recommended video:
Guided course
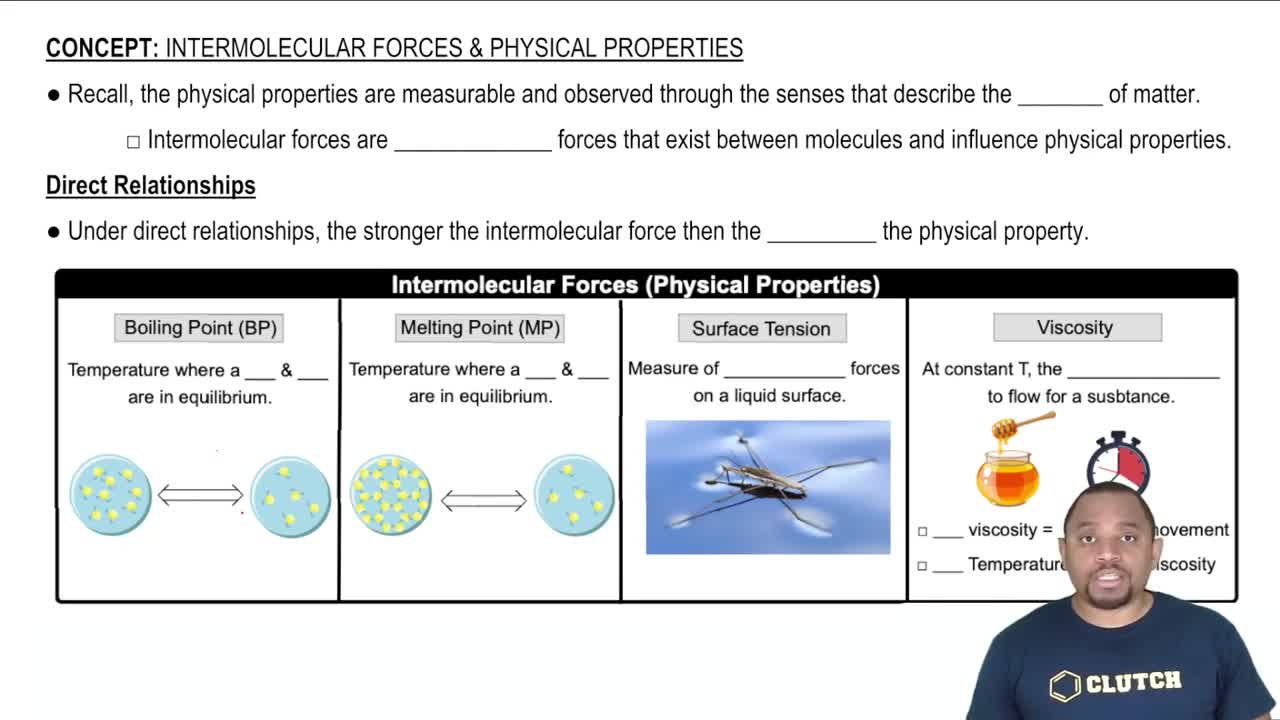
Intermolecular Forces and Properties
Adhesive Forces
Adhesive forces are the attractive forces between molecules of different substances. When a liquid comes into contact with a surface, adhesive forces determine how well the liquid spreads or wets the surface. In the case of water on a nonpolar surface, the adhesive forces are typically weaker than the cohesive forces, leading to minimal wetting and the formation of droplets.
Recommended video:
Guided course
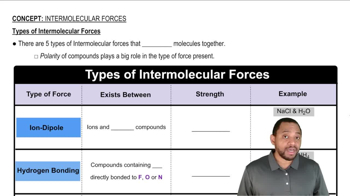
Types of Intermolecular Forces
Wetting and Contact Angle
Wetting refers to the ability of a liquid to maintain contact with a solid surface, influenced by the balance of cohesive and adhesive forces. The contact angle is a measure of this wetting; a smaller angle indicates better wetting. On nonpolar surfaces, water tends to form a larger contact angle, indicating poor wetting, which is crucial for interpreting the diagrams in the question.
Recommended video:
Guided course
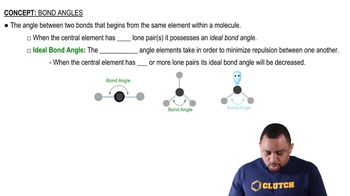
Bond Angles
Related Practice
Textbook Question
Textbook Question
(c) Why do substances with high surface tension also tend to have high viscosities?
Textbook Question
Based on their composition and structure, list CH2Cl2, CH3CH2CH3, and CH3CH2OH in order of (a) increasing intermolecular forces (c) increasing surface tension
Textbook Question
The boiling points, surface tensions, and viscosities of water and several alcohols are listed in the following table:
b. How do you explain the fact that propanol and ethylene glycol have similar molecular weights (60 vs. 62 amu), yet the viscosity of ethylene glycol is more than 10 times larger than propanol?
Textbook Question
Name the phase transition in each of the following situations and indicate whether it is exothermic or endothermic: (c) Rubbing alcohol in an open container slowly disappears.
