A sample of 3.00 g of SO2(g) originally in a 5.00-L vessel at 21 °C is transferred to a 10.0-L vessel at 26 °C. A sample of 2.35 g of N2(g) originally in a 2.50-L vessel at 20 °C is transferred to this same 10.0-L vessel. (a) What is the partial pressure of SO2(g) in the larger container? (b) What is the partial pressure of N2(g) in this vessel?

WF6 is one of the heaviest known gases. How much slower is the root-mean-square speed of WF6 than He at 300 K?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Root-Mean-Square Speed
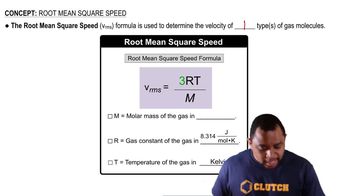
Molar Mass

Kinetic Molecular Theory

Determine whether each of the following changes will increase, decrease, or not affect the rate with which gas molecules collide with the walls of their container: (a) increasing the volume of the container (b) increasing the temperature (c) increasing the molar mass of the gas
Indicate which of the following statements regarding the kinetic-molecular theory of gases are correct. (a) The average kinetic energy of a collection of gas molecules at a given temperature is proportional to m1/2. (b) The gas molecules are assumed to exert no forces on each other. (c) All the molecules of a gas at a given temperature have the same kinetic energy. (d) The volume of the gas molecules is negligible in comparison to the total volume in which the gas is contained. (e) All gas molecules move with the same speed if they are at the same temperature.
You have an evacuated container of fixed volume and known mass and introduce a known mass of a gas sample. Measuring the pressure at constant temperature over time, you are surprised to see it slowly dropping. You measure the mass of the gas-filled container and find that the mass is what it should be—gas plus container—and the mass does not change over time, so you do not have a leak. Suggest an explanation for your observations.
The temperature of a 5.00-L container of N2 gas is increased from 20 °C to 250 °C. If the volume is held constant, predict qualitatively how this change affects the following: (a) the average kinetic energy of the molecules.
The temperature of a 5.00-L container of N2 gas is increased from 20 °C to 250 °C. If the volume is held constant, predict qualitatively how this change affects the following: (b) the rootmean-square speed of the molecules. (c) the strength of the impact of an average molecule with the container walls. (d) the total number of collisions of molecules with walls per second.
