Calculate the pressure that CCl4 will exert at 80 °C if 1.00 mol occupies 33.3 L, assuming that (a) CCl4 obeys the ideal-gas equation (b) CCl4 obeys the van der Waals equation. (Values for the van der Waals constants are given in Table 10.3.)

A 6.0-L tank is filled with helium gas at a pressure of 2 MPa. How many balloons (each 2.00 L) can be inflated to a pressure of 101.3 kPa, assuming that the temperature remains constant and that the tank cannot be emptied below 101.3 kPa?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Ideal Gas Law

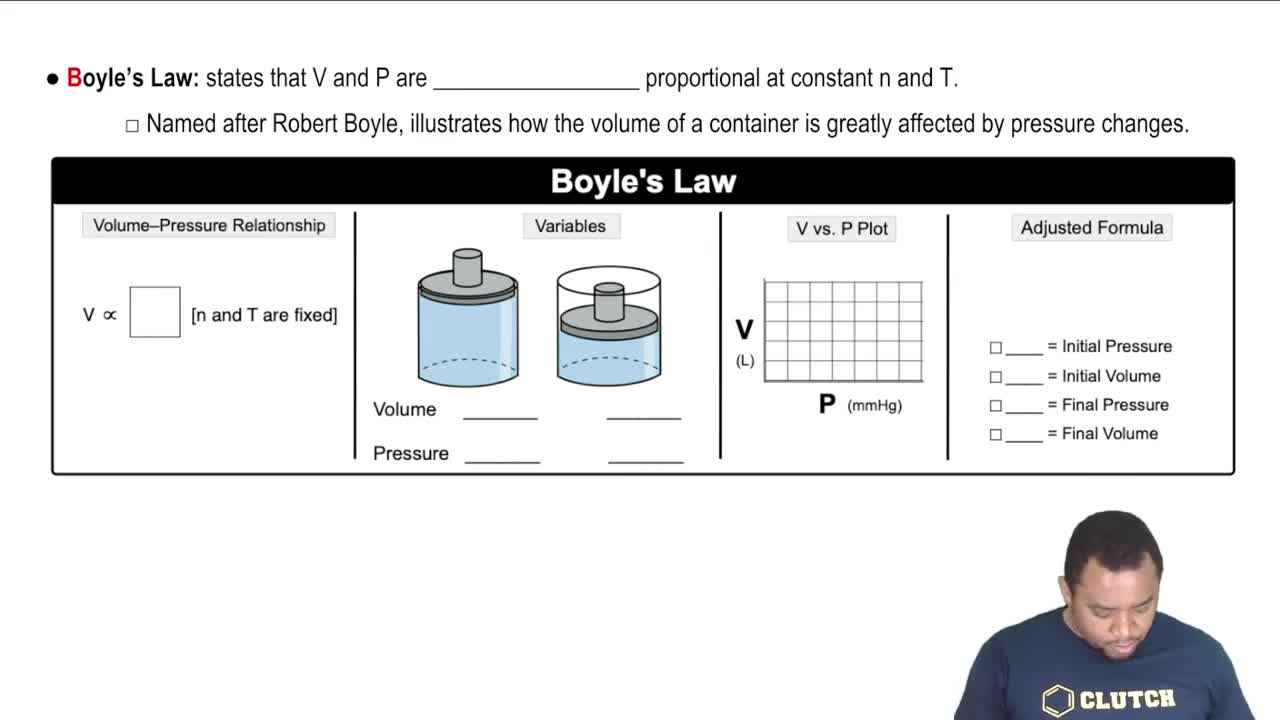
Boyle's Law
Gas Stoichiometry

Table 10.3 shows that the van der Waals b parameter has units of L/mol. This means that we can calculate the sizes of atoms or molecules from the b parameter. Refer back to the discussion in Section 7.3. Is the van der Waals radius we calculate from the b parameter of Table 10.3 more closely associated with the bonding or nonbonding atomic radius discussed there? Explain.
A gas bubble with a volume of 1.0 mm3 originates at the bottom of a lake where the pressure is 3.0 atm. Calculate its volume when the bubble reaches the surface of the lake where the pressure is 730 torr, assuming that the temperature does not change.
Carbon dioxide, which is recognized as the major contributor to global warming as a “greenhouse gas,” is formed when fossil fuels are combusted, as in electrical power plants fueled by coal, oil, or natural gas. One potential way to reduce the amount of CO2 added to the atmosphere is to store it as a compressed gas in underground formations. Consider a 1000-megawatt coal-fired power plant that produces about 6×106 tons of CO2 per year. a. Assuming ideal-gas behavior, 1.00 atm, and 27°C, calculate the volume of CO2 produced by this power plant.
Nickel carbonyl, Ni(CO)4, is one of the most toxic substances known. The present maximum allowable concentration in laboratory air during an 8-h workday is 1 ppb (parts per billion) by volume, which means that there is one mole of Ni(CO)4 for every 109 moles of gas. Assume 24°C and 1.00 atm pressure. What mass of Ni(CO)4 is allowable in a laboratory room that is 12ft×20ft×9ft?
Consider the arrangement of bulbs shown in the drawing. Each of the bulbs contains a gas at the pressure shown. What is the pressure of the system when all the stopcocks are opened, assuming that the temperature remains constant? (We can neglect the volume of the capillary tubing connecting the bulbs.)
