An aerosol spray can with a volume of 250 mL contains 2.30 g of propane gas (C3H8) as a propellant. c. The can’s label says that exposure to temperatures above 130°F may cause the can to burst. What is the pressure in the can at this temperature?

Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 gas is 8.70 L at 895 torr and 24°C. b. What volume will the Cl2 occupy at STP?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Ideal Gas Law

Standard Temperature and Pressure (STP)
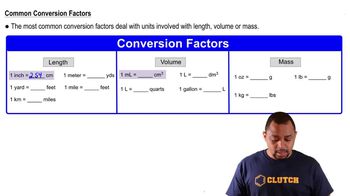
Gas Volume Conversion
A 35.1 g sample of solid CO2 (dry ice) is added to a container at a temperature of 100 K with a volume of 4.0 L. If the container is evacuated (all of the gas is removed), sealed, and then allowed to warm to room temperature (𝑇=298 K) so that all of the solid CO2 is converted to a gas, what is the pressure inside the container?
A 334-mL cylinder for use in chemistry lectures contains 5.225 g of helium at 23°C. How many grams of helium must be released to reduce the pressure to 75 atm assuming ideal-gas behavior?
Chlorine is widely used to purify municipal water supplies and to treat swimming pool waters. Suppose that the volume of a particular sample of Cl2 gas is 8.70 L at 895 torr and 24°C. c. At what temperature will the volume be 15.00 L if the pressure is 8.76×102 torr?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 55.0 gallons that contains O2 gas at a pressure of 16,500 kPa at 23°C. b. What volume would the gas occupy at STP?
Many gases are shipped in high-pressure containers. Consider a steel tank whose volume is 55.0 gallons that contains O2 gas at a pressure of 16,500 kPa at 23°C. c. At what temperature would the pressure in the tank equal 150.0 atm?
