Indicate which of the following are exact numbers: (a) the mass of a 32-oz bag of sugar, (b) the number of students in your chemistry class, (c) the temperature of the surface of the Sun, (d) the mass of a postage stamp, (e) the number of milliliters in a cubic meter of water, (f) the average height of NBA basketball players.
Ch.1 - Introduction: Matter, Energy, and Measurement

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 1, Problem 45e
What is the number of significant figures in each of the following measured quantities? e. 3.50×103 cm3
 Verified step by step guidance
Verified step by step guidance1
Identify the significant figures in the number 3.50.
Recognize that all non-zero digits are significant.
Note that zeros between non-zero digits are also significant.
Understand that zeros to the right of a decimal point and at the end of a number are significant.
Conclude that the number 3.50 has three significant figures.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Significant Figures
Significant figures are the digits in a number that contribute to its precision. This includes all non-zero digits, any zeros between significant digits, and trailing zeros in the decimal portion. Understanding significant figures is crucial for accurately reporting measurements and ensuring that calculations reflect the precision of the data.
Recommended video:
Guided course

Significant Figures Example
Scientific Notation
Scientific notation is a way of expressing numbers that are too large or too small to be conveniently written in decimal form. It is represented as a product of a number between 1 and 10 and a power of ten. For example, 3.50×10³ cm³ indicates that the number 3.50 is multiplied by 1000, which helps in identifying significant figures more easily.
Recommended video:
Guided course

Standard Notation to Scientific Notation
Rules for Counting Significant Figures
There are specific rules for counting significant figures in a number. For instance, all non-zero digits are significant, any zeros between significant digits are significant, and trailing zeros in a decimal number are also significant. Applying these rules helps determine the number of significant figures in a measurement, which is essential for scientific accuracy.
Recommended video:
Guided course
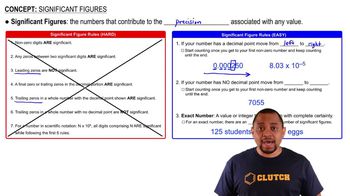
Significant Figures Rules
Related Practice
Textbook Question
Textbook Question
What is the number of significant figures in each of the following measured quantities? b. 32.40 s
Textbook Question
What is the number of significant figures in each of the following measured quantities? d. 0.00404 L
Textbook Question
Indicate the number of significant figures in each of the following measured quantities: e. 12.8690 g
Textbook Question
Round each of the following numbers to four significant figures and express the result in standard exponential notation: (a) 102.53070
1
views
Textbook Question
Round each of the following numbers to four significant figures and express the result in standard exponential notation: (e) −0.0357202.
1
views
