The following data were collected for the desturction of O3 by H (O3 + H → O2 + OH) at very low concentrations (b) Calculate the rate constant

The degradation of CF3CH2F (an HFC) by OH radicals in the troposphere is first order in each reactant and has a rate constant of k = 1.6x10^8 M-1s-1 at 4 °C. If the tropospheric concentrations of OH and CF3CH2F are 8.1x10^5 and 6.3x10^8 molecules/cm3, respectively, what is the reaction rate at this temperature in M/s?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
First-Order Reactions
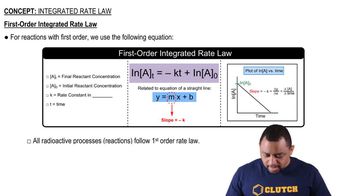
Rate Constant (k)

Concentration Units and Conversion

The Henry's law constant for CO2 in water at 25 °C is 3.1x10^-2 M atm-1. (a) What is the soubility of CO2 in water at this temperature if the soltuion is in contact with air at normal atmospheric pressure?
The precipitation of Al(OH)3 (Ksp) = 1.3⨉10-33) is sometimes used ot purify water. (a) Estimate the pH at which precipitation of Al(OH)3 will begin if 5.0 lb of Al2(SO4)3 is added to 2000 gal of water
The valuable polymer polyurethane is made by a condensa- tion reaction of alcohols (ROH) with compounds that con- tain an isocyanate group (RNCO). Two reactions that can generate a urethane monomer are shown here: (i)
(ii)
(c) If you wanted to promote the formation of the isocyanate intermediate in each reaction, what could you do, using Le Châtelier's principle?
