Textbook Question
In the following three instances, which choice is greener in a chemical process? Explain. (a) A reaction that can be run at 350 K for 12 h without a catalyst or one that can be run at 300 K for 1 h with a reusable catalyst.
1
views

 Verified step by step guidance
Verified step by step guidance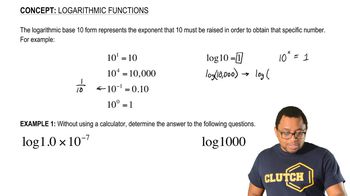


In the following three instances, which choice is greener in a chemical process? Explain. (a) A reaction that can be run at 350 K for 12 h without a catalyst or one that can be run at 300 K for 1 h with a reusable catalyst.
Suppose that on another planet the atmosphere consists of 17% Kr, 38% CH4, and 45% O2. What is the average molar mass at the surface? What is the average molar mass at an altitude at which all the O2 is photodissociated?
Show how Equations 18.7 and 18.9 can be added to give Equation 18.10.