Textbook Question
The following data were collected for the desturction of O3 by H (O3 + H → O2 + OH) at very low concentrations (b) Calculate the rate constant

 Verified step by step guidance
Verified step by step guidance
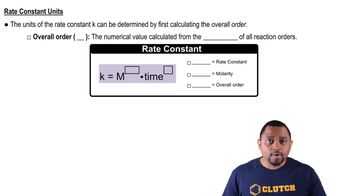

The following data were collected for the desturction of O3 by H (O3 + H → O2 + OH) at very low concentrations (b) Calculate the rate constant
The Henry's law constant for CO2 in water at 25 °C is 3.1x10^-2 M atm-1. (a) What is the soubility of CO2 in water at this temperature if the soltuion is in contact with air at normal atmospheric pressure?