Ethyl propanoate, CH3CH2COOCH2CH3, gives a fruity pineapple-like smell. (e) What are the approximate bond angles around each carbon atom in the molecule?

The O-H bond lengths in the water molecule 1H2O are 96 pm, and the H-O-H angle is 104.5°. The dipole moment of the water molecule is 1.85 D. (c) Compare your answer from part (b) to the dipole moments of the hydrogen halides (Table 8.3). Is your answer in accord with the relative electronegativity of oxygen?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Dipole Moment
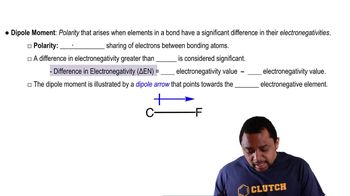
Electronegativity

Molecular Geometry

An AB5 molecule adopts the geometry shown here. (b) Do you think there are any nonbonding electron pairs on atom A?
An AB5 molecule adopts the geometry shown here. (c) Suppose the B atoms are halogen atoms. Of which group in the periodic table is atom A a member: (i) Group 15, (ii) Group 16, (iii) Group 17, (iv) Group 18, or (v) More information is needed?
The O¬H bond lengths in the water molecule 1H2O2 are 96 pm, and the H¬O¬H angle is 104.5°. The dipole moment of the water molecule is 1.85 D. (b) Calculate the magnitude of the bond dipole of the O¬H bonds. (Note: You will need to use vector addition to do this.)
a) Predict the electron-domain geometry around the central S atom in SF2, SF4, and SF6.
(b) The anion IO4- has a tetrahedral structure: three oxygen atoms form double bonds with the central iodine atom and one oxygen atom which carries a negative charge forms a single bond. Predict the molecular geometry of IO65-.
