The figure that follows contains ball-and-stick drawings of three possible shapes of an AF4 molecule. (a) For each shape, give the electron-domain geometry on which the molecular geometry is based. iii.
Ch.9 - Molecular Geometry and Bonding Theories

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 9, Problem 28c
The figure that follows contains ball-and-stick drawings of three possible shapes of an AF4 molecule. (c) Which of the following elements will lead to an AF4 molecule with the shape in (iii): Be, C, S, Se, Si, Xe? i.

ii.
![]()
iii.
![]()
 Verified step by step guidance
Verified step by step guidance1
Identify the shape of the AF4 molecule in the given image. The shape shown is a seesaw geometry.
Determine the electron domain geometry that leads to a seesaw shape. A seesaw shape arises from a trigonal bipyramidal electron domain geometry with one lone pair.
Count the total number of valence electrons for each element in the list: Be, C, S, Se, Si, Xe.
Determine which of these elements can form a molecule with 5 electron domains (4 bonding pairs and 1 lone pair).
Select the element that fits the criteria. The element must have 6 valence electrons to form a seesaw shape with 4 fluorine atoms and 1 lone pair.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs of electrons around the central atom, which influences the shape of the molecule. Common geometries include linear, trigonal planar, tetrahedral, and octahedral, each corresponding to specific bond angles and arrangements.
Recommended video:
Guided course

Molecular Geometry with Two Electron Groups
Valence Shell Electron Pair Repulsion (VSEPR) Theory
VSEPR theory is a model used to predict the geometry of individual molecules based on the repulsion between electron pairs in the valence shell of the central atom. According to this theory, electron pairs will arrange themselves as far apart as possible to minimize repulsion, leading to specific molecular shapes that can be identified based on the number of bonding and non-bonding electron pairs.
Recommended video:
Guided course
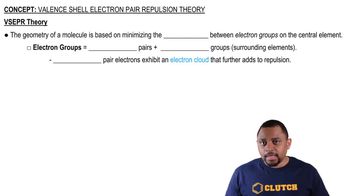
Valence Shell Electron Pair Repulsion Theory
Hybridization
Hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals that can accommodate bonding in a molecule. This process helps explain the geometry of molecules by allowing for the formation of equivalent bonds. For example, in a tetrahedral geometry, the central atom undergoes sp3 hybridization, resulting in four equivalent hybrid orbitals that form bonds with surrounding atoms.
Recommended video:
Guided course

Hybridization
Related Practice
Textbook Question
2
views
Textbook Question
The figure that follows contains ball-and-stick drawings of three possible shapes of an AF4 molecule. (a) For each shape, give the electron-domain geometry on which the molecular geometry is based. i.
4
views
Textbook Question
The figure that follows contains ball-and-stick drawings of three possible shapes of an AF4 molecule. (a) For each shape, give the electron-domain geometry on which the molecular geometry is based. ii.
1
views
Textbook Question
Give the approximate values for the indicated bond angles in the following molecules: (a)
Textbook Question
Give the approximate values for the indicated bond angles in the following molecules: (c)
Textbook Question
Give the approximate values for the indicated bond angles in the following molecules: (a)
