(c) With what neutral homonuclear diatomic molecules are the NO+ and NO- ions isoelectronic (same number of electrons)? With what neutral homonuclear diatomic molecule is the NO- ion isoelectronic (same number of electrons)?

Consider the molecular orbitals of the P2 molecule. Assume that the MOs of diatomics from the third row of the periodic table are analogous to those from the second row. (a) Which valence atomic orbitals of P are used to construct the MOs of P2?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Molecular Orbitals (MOs)
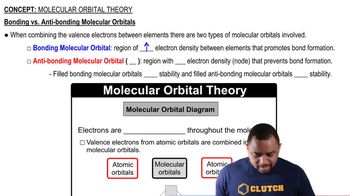
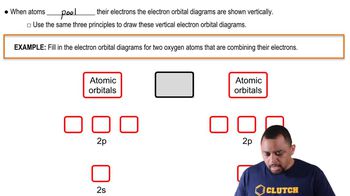
Valence Atomic Orbitals
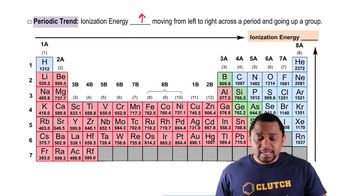
Periodic Trends in Orbital Energy
Determine the electron configurations for CN+, CN, and CN-. (b) Which species, if any, has unpaired electrons?
(a) The nitric oxide molecule, NO, readily loses one electron to form the NO+ ion. Which of the following is the best explanation of why this happens: (i) Oxygen is more electronegative than nitrogen, (ii) The highest energy electron in NO lies in a π2p* molecular orbital, or (iii) The π2p* MO in NO is completely filled.
Consider the molecular orbitals of the P2 molecule. Assume that the MOs of diatomics from the third row of the periodic table are analogous to those from the second row. (c) For the P2 molecule, how many electrons occupy the MO in the figure?
The iodine bromide molecule, IBr, is an interhalogen compound. Assume that the molecular orbitals of IBr are analogous to the homonuclear diatomic molecule F2. (a) Which valence atomic orbitals of I and of Br are used to construct the MOs of IBr?
The iodine bromide molecule, IBr, is an interhalogen compound. Assume that the molecular orbitals of IBr are analogous to the homonuclear diatomic molecule F2. (c) One of the valence MOs of IBr is sketched here. Why are the atomic orbital contributions to this MO different in size?
