In ozone, O3, the two oxygen atoms on the ends of the molecule are equivalent to one another. (d) How many electrons are delocalized in the p system of ozone?

Butadiene, C4H6, is a planar molecule that has the following carbon–carbon bond lengths:

(b) From left to right, what is the hybridization of each carbon atom in butadiene?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
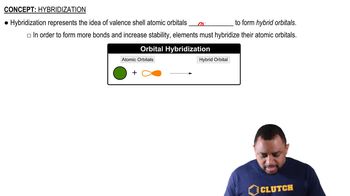
Hybridization
Bond Lengths

Planarity of Molecules
Butadiene, C4H6, is a planar molecule that has the following carbon–carbon bond lengths:
(a) Predict the bond angles around each of the carbon atoms and sketch the molecule.
Butadiene, C4H6, is a planar molecule that has the following carbon–carbon bond lengths:
(c) The middle C¬C bond length in butadiene (1.48 Å) is a little shorter than the average C¬C single bond length (1.54 Å). Does this imply that the middle C¬C bond in butadiene is weaker or stronger than the average C¬C single bond?
The structure of borazine, B3N3H6, is a six-membered ring of alternating B and N atoms. There is one H atom bonded to each B and to each N atom. The molecule is planar. (a) Write a Lewis structure for borazine in which the formal charge on every atom is zero.
The structure of borazine, B3N3H6, is a six-membered ring of alternating B and N atoms. There is one H atom bonded to each B and to each N atom. The molecule is planar. (c) What are the formal charges on the atoms in the Lewis structure from part (b)? Given the electronegativities of B and N, do the formal charges seem favorable or unfavorable? What are the formal charges on the atoms in the Lewis structure from part (b)?
