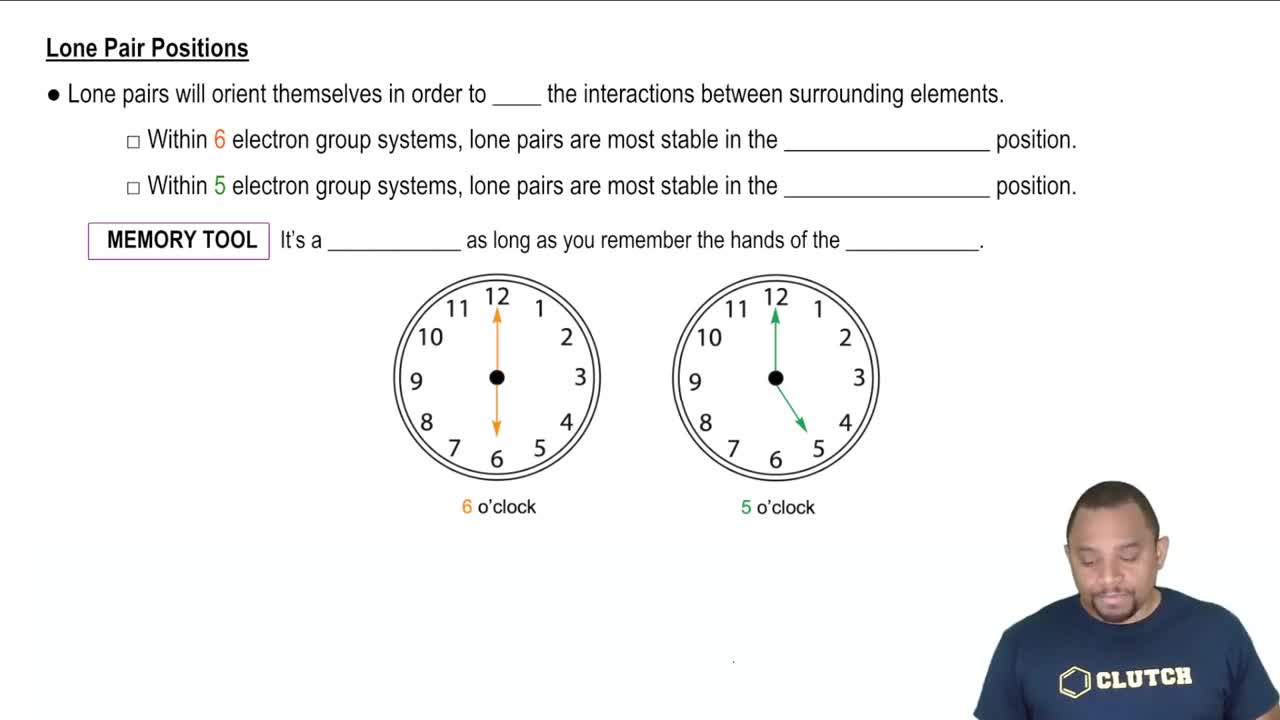
Textbook Question
How does a trigonal pyramid differ from a tetrahedron so far as molecular geometry is concerned?
4
views

 Verified step by step guidance
Verified step by step guidance


Describe the bond angles to be found in each of the following molecular structures: (a) trigonal planar, (b) tetrahedral, (c) octahedral, (d) linear.
(a) An AB6 molecule has no lone pairs of electrons on the A atom. What is its molecular geometry? (c) For the AB4 molecule in part (b), predict the molecular geometry.
Would you expect the nonbonding electron-pair domain in NCl3 to be greater or smaller in size than the corresponding one in PCl3?
In which of the following molecules can you confidently predict the bond angles about the central atom, and for which would you be a bit uncertain? Explain in each case. (a) H2S, (b) BCl3, (c) CH3I, (d) CBr4, (e) TeBr4.